The ionization-constant expression for a solution of a weak acid can be written as:
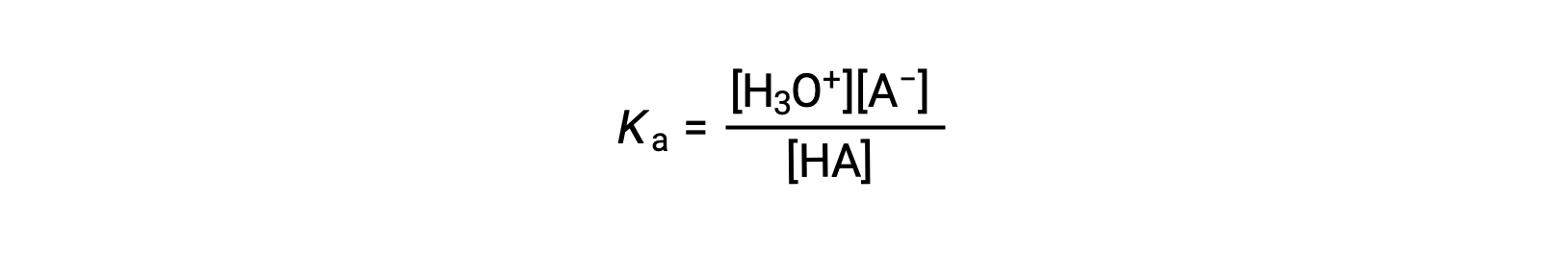
Rearranging to solve for [H3O+] yields:
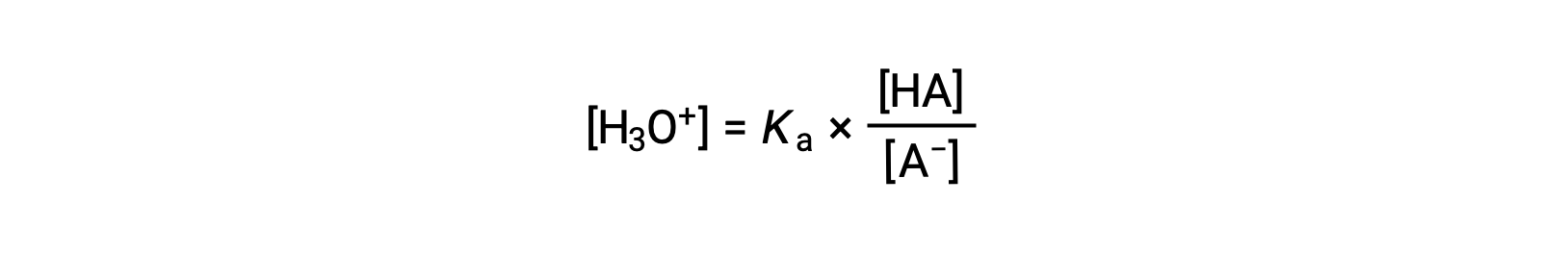
Taking the negative logarithm of both sides of this equation gives
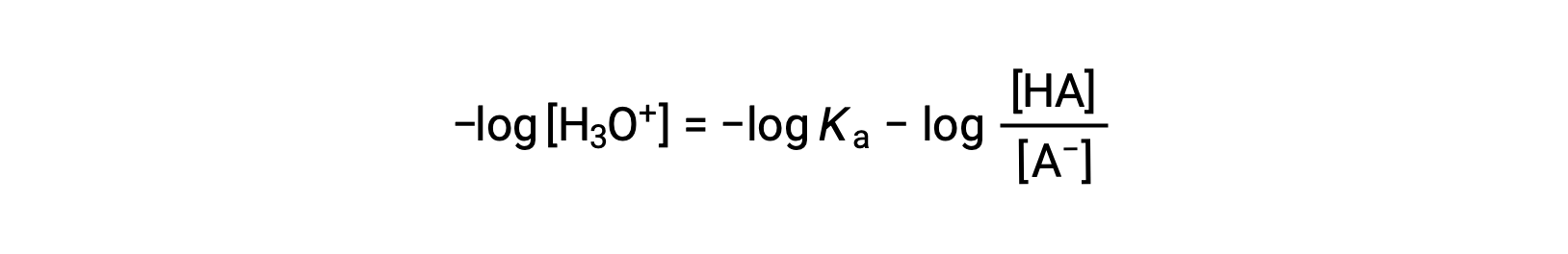
which can be written as
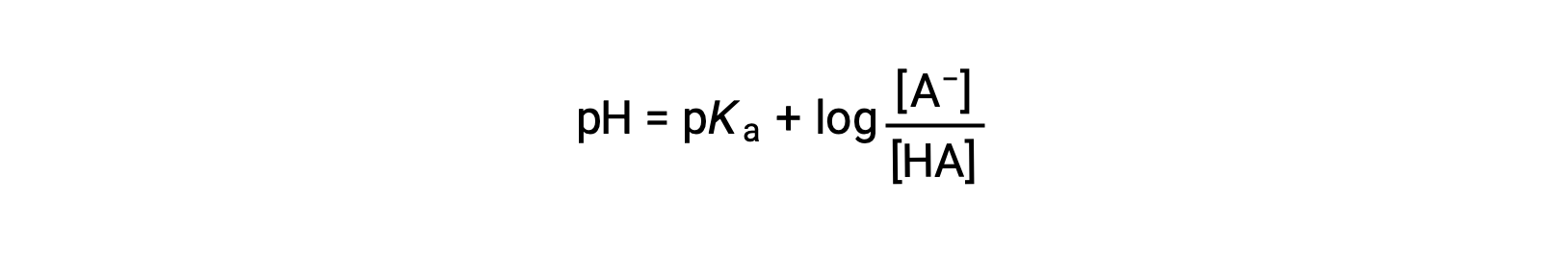
where pKa is the negative of the logarithm of the ionization constant of the weak acid (pKa = −log Ka). This equation relates the pH, the ionization constant of a weak acid, and the concentrations of the weak conjugate acid-base pair in a buffered solution. Scientists often use this expression, called the Henderson-Hasselbalch equation, to calculate the pH of buffer solutions. It is important to note that the “x is small” assumption must be valid to use this equation.
Lawrence Joseph Henderson and Karl Albert Hasselbalch
Lawrence Joseph Henderson (1878–1942) was an American physician, biochemist and physiologist, to name only a few of his many pursuits. He obtained a medical degree from Harvard and then spent 2 years studying in Strasbourg, then a part of Germany, before returning to take a lecturer position at Harvard. He eventually became a professor at Harvard and worked there his entire life. He discovered that the acid-base balance in human blood is regulated by a buffer system formed by the dissolved carbon dioxide in blood. He wrote an equation in 1908 to describe the carbonic acid-carbonate buffer system in blood. Henderson was broadly knowledgeable; in addition to his important research on the physiology of blood, he also wrote on the adaptations of organisms and their fit with their environments, on sociology and on university education. He also founded the Fatigue Laboratory at the Harvard Business School, which examined human physiology with a specific focus on work in industry, exercise, and nutrition.
In 1916, Karl Albert Hasselbalch (1874–1962), a Danish physician and chemist, shared authorship in a paper with Christian Bohr in 1904 that described the Bohr effect, which showed that the ability of hemoglobin in the blood to bind with oxygen was inversely related to the acidity of the blood and the concentration of carbon dioxide. The pH scale was introduced in 1909 by another Dane, Sørensen, and in 1912, Hasselbalch published measurements of the pH of blood. In 1916, Hasselbalch expressed Henderson’s equation in logarithmic terms, consistent with the logarithmic scale of pH, and thus the Henderson-Hasselbalch equation was born.
This text is adapted from Openstax, Chemistry 2e, Section 14.6: Buffers.
From Chapter 16:

Now Playing
16.3 : Henderson-Hasselbalch Equation
Acid-base and Solubility Equilibria
66.1K Views

16.1 : Common Ion Effect
Acid-base and Solubility Equilibria
39.4K Views

16.2 : Buffers
Acid-base and Solubility Equilibria
160.8K Views

16.4 : Calculating pH Changes in a Buffer Solution
Acid-base and Solubility Equilibria
50.5K Views

16.5 : Buffer Effectiveness
Acid-base and Solubility Equilibria
46.9K Views

16.6 : Titration Calculations: Strong Acid - Strong Base
Acid-base and Solubility Equilibria
27.4K Views

16.7 : Titration Calculations: Weak Acid - Strong Base
Acid-base and Solubility Equilibria
41.4K Views

16.8 : Indicators
Acid-base and Solubility Equilibria
46.3K Views

16.9 : Titration of a Polyprotic Acid
Acid-base and Solubility Equilibria
91.9K Views

16.10 : Solubility Equilibria
Acid-base and Solubility Equilibria
48.2K Views

16.11 : Factors Affecting Solubility
Acid-base and Solubility Equilibria
31.6K Views

16.12 : Formation of Complex Ions
Acid-base and Solubility Equilibria
22.2K Views

16.13 : Precipitation of Ions
Acid-base and Solubility Equilibria
26.6K Views

16.14 : Qualitative Analysis
Acid-base and Solubility Equilibria
16.2K Views

16.15 : Acid-Base Titration Curves
Acid-base and Solubility Equilibria
122.1K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved