A buffer can prevent a sudden drop or increase in the pH of a solution after the addition of a strong acid or base up to its buffering capacity; however, such addition of a strong acid or base does result in the slight pH change of the solution. The small pH change can be calculated by determining the resulting change in the concentration of buffer components, i.e., a weak acid and its conjugate base or vice versa. The concentrations obtained using these stoichiometric calculations can be used to determine the solution’s final pH using the Henderson-Hasselbalch equation or an ICE table.
For example, a buffered solution contains 0.65 mol of formic acid and sodium formate. As the concentration of the weak acid and its conjugate base is the same here, the solution’s pH is equal to the pKa of the weak acid, which is 3.74 in this case. If 0.05 mol HNO3 is added into this solution, the resultant changes in the concentration of the formic acid and sodium formate can be determined by stoichiometric calculations as shown in the table below.
| H+ (aq) | HCOO− (aq) | HCOOH (aq) | |
| Before addition (M) | ~0.00 mol | 0.65 mol | 0.65 mol |
| Addition (M) | 0.050 mol | - | - |
| After addition (M) | ~0.00 mol | 0.60 mol | 0.70 mol |
The solution’s final pH can then be determined by plugging in changed concentrations of formic acid and sodium formate into the Henderson-Hasselbalch equation.
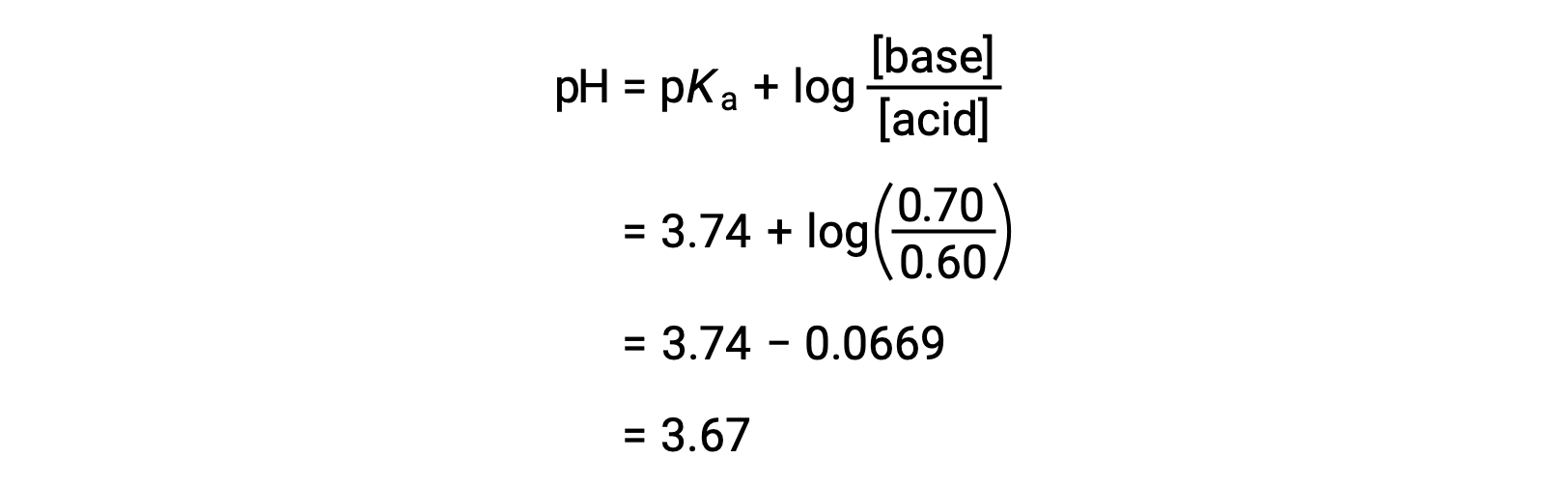
Thus, the addition of 0.05 mol of HNO3 reduces the pH of the solution from 3.74 to 3.67.
Similarly, if 0.10 mol NaOH is added into the same solution, the resultant changes in the concentration of the formic acid and sodium formate can be determined by stoichiometric calculations as shown in the table below.
| OH− (aq) | HCOOH (aq) | HCOO− (aq) | H2O (l) | |
| Before addition (M) | ~0.00 mol | 0.65 mol | 0.65 mol | - |
| Addition (M) | 0.10 mol | - | - | - |
| After addition (M) | ~0.00 mol | 0.55 mol | 0.75 mol | - |
The final pH of the solution can then be determined by plugging in changed concentrations of formic acid and sodium formate into the Henderson-Hasselbalch equation.
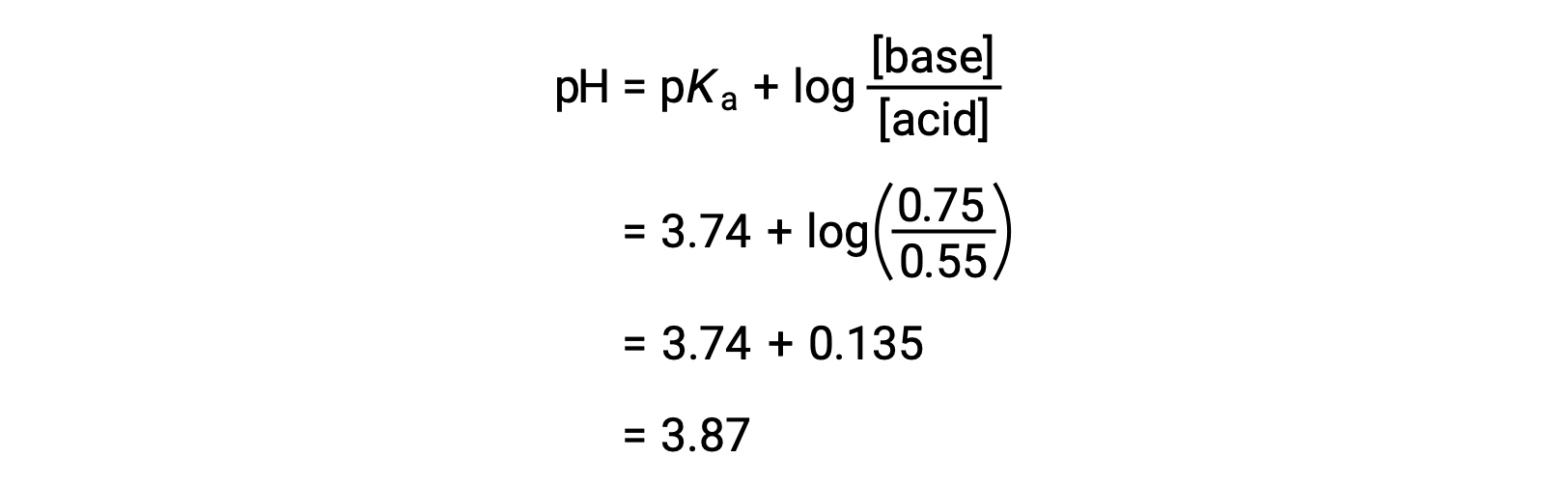
Thus, the addition of 0.10 mol NaOH increases the pH of the solution from 3.74 to 3.87.
From Chapter 16:

Now Playing
16.4 : Calculating pH Changes in a Buffer Solution
Acid-base and Solubility Equilibria
51.0K Views

16.1 : Common Ion Effect
Acid-base and Solubility Equilibria
39.9K Views

16.2 : Buffers
Acid-base and Solubility Equilibria
161.7K Views

16.3 : Henderson-Hasselbalch Equation
Acid-base and Solubility Equilibria
66.8K Views

16.5 : Buffer Effectiveness
Acid-base and Solubility Equilibria
47.3K Views

16.6 : Titration Calculations: Strong Acid - Strong Base
Acid-base and Solubility Equilibria
27.9K Views

16.7 : Titration Calculations: Weak Acid - Strong Base
Acid-base and Solubility Equilibria
42.3K Views

16.8 : Indicators
Acid-base and Solubility Equilibria
47.0K Views

16.9 : Titration of a Polyprotic Acid
Acid-base and Solubility Equilibria
94.9K Views

16.10 : Solubility Equilibria
Acid-base and Solubility Equilibria
49.6K Views

16.11 : Factors Affecting Solubility
Acid-base and Solubility Equilibria
32.4K Views

16.12 : Formation of Complex Ions
Acid-base and Solubility Equilibria
22.6K Views

16.13 : Precipitation of Ions
Acid-base and Solubility Equilibria
27.0K Views

16.14 : Qualitative Analysis
Acid-base and Solubility Equilibria
17.9K Views

16.15 : Acid-Base Titration Curves
Acid-base and Solubility Equilibria
123.8K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved