Method Article
Lumbar Intrathecal Injection of Gene Therapy Vectors for Central Nervous System Targeting in Mice and Rats
* These authors contributed equally
In This Article
Summary
A lumbar intrathecal injection represents a translationally relevant route of administration for delivering gene therapy to the central nervous system. This comprehensive standardized protocol for lumbar intrathecal injections in neonatal, juvenile, and adult mice and rats aims to guide researchers in adopting this technique for preclinical gene therapy studies.
Abstract
One method to target the central nervous system for treating neurological diseases involves utilizing the lumbar intrathecal route of administration. This approach bypasses the blood-brain barrier to directly access the cerebrospinal fluid and preferentially target cells within the central nervous system. Multiple published preclinical studies employing the lumbar intrathecal injection route have contributed to the development of gene therapy clinical trials; however, the described protocols are variable and dispersed across multiple resources. Here, a comprehensive set of protocols for lumbar intrathecal injections in neonatal, juvenile, and adult mice and rats for preclinical gene therapy studies is presented. With proper training, this injection technique can be performed quickly and reliably. In addition to detailing the injection protocol at each developmental stage, associated parameters, such as injection volume, that can influence study outcomes are discussed. To demonstrate the application of lumbar intrathecal injections for targeting the central nervous system, the expression of adeno-associated virus serotype 9 in the brain, spinal cord, and peripheral tissues is presented following a successful or unsuccessful injection.
Introduction
A challenge in treating neurological diseases that require global central nervous system (CNS) delivery but are otherwise good candidates for gene therapy has been largely attributed to inefficient targeting of the CNS and relevant cell types1. A substantial amount of research is ongoing to optimize global CNS cell and tissue targeting by engineering delivery vehicles1,2. However, reasonably widespread vector delivery can still be achieved with current gene therapy vector technology, using certain combinations of viral vectors and routes of administration3,4. The current gold standard for obtaining widespread CNS delivery from a one-time treatment is to use adeno-associated virus serotype 9 (AAV9) together with a direct injection into the cerebrospinal fluid (CSF).
There are three typical routes of administration for direct CSF injections: lumbar intrathecal (IT), intracerebroventricular (ICV), and intracisternal (ICM)5. Each of these routes of administration results in different biodistribution patterns in the CNS and peripheral tissues, but they all have the benefit of bypassing the blood-brain barrier (BBB) to reach the cells in the CNS that contribute to neurological disease pathology and phenotypes6. The lumbar IT injection is the standard for clinical drug delivery use in humans, as the clinical procedure is routine and straightforward, with less invasiveness compared to ICV and ICM injections.
The lumbar IT injection is an established technique that is readily used in the anesthetics and analgesic fields, with the first paper published in 18857. The first protocol for lumbar IT injections in adult mice was published in 19808, and it has since been widely adopted and reviewed9. Slight adjustments or improvements to these protocols have been made10,11,12, including a product-conserving technique13. Protocols for lumbar IT injections in adult rats were also first published in 1976, with catheterization for chronic administration14 and direct injection for one-time treatments15. More recently, groups have published protocols for lumbar IT injections in neonatal or juvenile mice and rats16,17.
Wide adoption and validation of this technique to bypass the BBB and target cells in the CNS has led to multiple successful gene therapy preclinical and clinical studies for the treatment of neurological diseases. Positive efficacy and safety data in mice, rats, and non-human primates modeling neurological diseases have piqued excitement and interest around the potential of clinical benefit for these diseases18,19,20,21,22,23. A handful of these studies are now in clinical trials (for example, clinicaltrials.gov identifiers NCT02362438, NCT04737460, NCT03381729, and NCT05518188)3,6. In this article, a simple protocol for lumbar IT injections in mice and rats of different ages is described, without removing CSF, that can be adopted for translational gene therapy projects. This protocol is similar to already-available protocols that are widely adopted; however, there is value in citing these relevant protocols in one place for easy access and reference, along with the accompanying video visuals. This protocol explains the injection for neonatal mice and rats at postnatal day (P) 0-1 and juvenile mice and rats <P10, as well as for mice and rats >P21, with representative results from a successful and unsuccessful lumbar IT injection at P1 in mice. In the discussion, common missteps and specific details that require careful attention while performing this procedure, as well as recommendations on how to practice these injections before starting a preclinical study, are addressed.
Protocol
The procedures described herein were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Texas Southwestern Medical Center. Wild-type C57BL6/J male and female mice, aged P1-P28, were used for protocols involving mice. Wild-type Sprague-Dawley male and female rats, aged P1-P56, were used for protocols involving rats. Aside from the survival surgery described in section 3, all other procedures are considered to cause only momentary discomfort and do not require the use of anesthetics or analgesics. Individuals should monitor laboratory animals for more than momentary discomfort and seek guidance from their IACUC and veterinary staff on the necessity of anesthetics and analgesics. Details of the reagents and equipment utilized are provided in the Table of Materials.
1. Lumbar IT injection of mice >P21
- Preparation
- Prepare the injection solutions, including the gene therapy vector at the desired concentration(s) and a control solution (usually the formulation buffer used in vector production). The injection solutions should be sterile and should remain sterile throughout the procedure.
NOTE: All solutions should be maintained on ice for the duration of the procedure. - Gather and sanitize all materials, including syringe and needle, pipette, and injection solution. Follow institutional guidelines regarding the use of biosafety cabinets and injectant exposure. The procedures described here were conducted in a class 2 biosafety cabinet.
- Using a microliter pipette, measure the desired volume of injection solution and transfer it to sterile paraffin film. An injection volume of 5 µL is commonly used and is treated as the target volume for adult mice >P21 (Table 1).
NOTE: The volume can be increased if necessary; however, animals should be monitored closely for adverse reactions to larger injection volumes. See the Discussion section for details on injection volume and adverse reactions. The solution can also be transferred directly from the pipette tip to a syringe, or calibrated gas-tight microliter syringes can be used to directly measure injection solution volume. - Draw the solution into a microliter syringe with a 30 G 0.5" needle, careful not to draw up any air bubbles.
NOTE: Needle gauge and length can affect skin penetration. When considering the size of the syringe to be used, the injection volume should not be less than 10% of the syringe capacity. For volumes greater than 50% of syringe capacity, ensure the plunger can easily be depressed with your index finger. If the plunger cannot be easily depressed, use a larger-capacity syringe.
- Prepare the injection solutions, including the gene therapy vector at the desired concentration(s) and a control solution (usually the formulation buffer used in vector production). The injection solutions should be sterile and should remain sterile throughout the procedure.
- Restraining
- Using the dominant hand, hold the conscious (unanesthetized) mouse by the tail on a paper towel. The paper towel will help restrain, calm, and prevent the mouse from biting. The mouse should be in the neutral prone position with the lateral aspect of the body facing you.
NOTE: It is not necessary to train or habituate mice to this restraint prior to the procedure. Mice may struggle due to restraint but should not elicit a specific pain response. If more than momentary pain responses are observed, administration of analgesics may be necessary; seek veterinary guidance. - With the non-dominant hand, fold a portion of the paper towel over the head and upper body of the mouse and firmly grasp the pelvic girdle between the non-dominant thumb and index finger. Ensure that the palm is cupped, resting around the head of the mouse. The mouse's iliac crest should be palpable and positioned in the center of the distal phalanx of each finger (Figure 1A,B).
NOTE: Restraint from hand or paper towel should not impair respiration. - Gently rotate the base of the tail to ensure proper alignment of the spine. The pelvis of the mouse should be square, such that the vertebral processes of the lumbar spine are perpendicular to the work surface.
- Swab the dorsal lumbar region of the mouse with a 70% alcohol prep pad.
- Part the hair ~2-6 mm cranial to the iliac crest to help visualize the injection location.
- Feel for the L4-L5 or L5-L6 intervertebral space, ~2-6 mm cranial to the iliac crest.
NOTE: The exact distance is variable depending on the size and age of the mouse.
- Using the dominant hand, hold the conscious (unanesthetized) mouse by the tail on a paper towel. The paper towel will help restrain, calm, and prevent the mouse from biting. The mouse should be in the neutral prone position with the lateral aspect of the body facing you.
- Injection
- Using the dominant hand, hold the center of the microliter syringe and position it perpendicular to the mouse spine at the L4-L5 or L5-L6 intervertebral space. Ensure that the plunger of the syringe can be depressed with your index finger without excessive movement of your hand. With the bevel of the needle facing the head of the mouse, puncture the skin over the intervertebral space.
- When the edges of the bone around the intervertebral space are felt at the tip of the needle, reduce the angle of the syringe to 30-45 degrees (Figure 1A).
- The needle should slip into the intervertebral space causing a sudden tail flick, and should be tightly clamped between the vertebrae.
- Taking care not to displace the syringe, depress the plunger steadily to deliver the solution within 10 s.
- Hold the syringe in place for 15-30 s after fully depressing the plunger to allow dissipation of the solution and prevent backflow.
- Smoothly and slowly withdraw the syringe from the mouse at the same 30-45-degree entry angle. As the needle is withdrawn, rotate the syringe to prevent backflow.
NOTE: Use caution not to advance the needle when rotating the syringe, as this may cause physical damage to the spinal cord. - Release the mouse from the restraint and return it to the home cage.
- Monitor the mouse after injection for abnormal ambulation, motor impairment, breathing abnormalities, and coordination. See the Discussion for additional details regarding adverse reactions.
- Clean equipment following the manufacturer's recommendations. For multiple injections, different syringes are recommended for each gene therapy vector and control solution.
NOTE: Removable needles can be cleaned, sterilized, and reused multiple times; however, dull needles should not be used as they can harm the animal and increase the likelihood of missed injections. A dull needle will not puncture the skin easily and should be discarded appropriately. If syringes are limited, it is advisable to complete all vehicle injections before proceeding to the gene therapy vector and to inject the lowest gene therapy dose before administering higher doses. - Follow institutional guidelines for sterilization of equipment between animals.
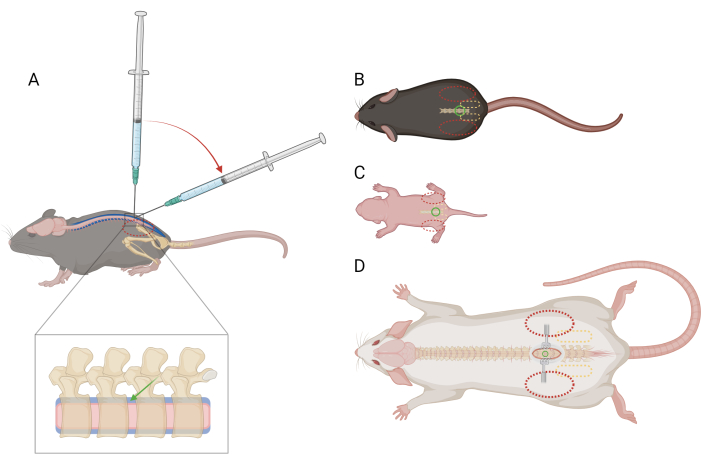
Figure 1: Schematic of finger and syringe placement for lumbar intrathecal injection in mice and rats. (A) Lateral view of a mouse >P21 showing needle placement and syringe angle transition during a lumbar IT injection. The dashed red oval indicates finger positioning over the mouse's iliac crest. An enlarged view of the spine shows the intrathecal space (blue) with approximate needle placement (green arrow) and the spinal cord (pink). Dorsal view of (B) a mouse >P21, (C) a mouse <P10, and (D) a rat >P21, with reference points for needle placement (green circle), pelvic girdle (dashed yellow oval), and finger placement (dashed red oval). The incision site and retractors are also depicted in (D). Please click here to view a larger version of this figure.
2. Lumbar IT injection of mice and rats <P10
- Preparation
- Follow step 1.1. The only exception is the injection volume in step 1.1.3.
NOTE: Recommended injection volumes for neonatal mice (P0-P1) should not exceed 3 µL, for juvenile mice (P5-P7) should not exceed 5 µL, and for juvenile mice (P10) should not exceed 10 µL. Recommended injection volumes for neonatal rats (P0-P1) should not exceed 5 µL, for juvenile rats (P5-P7) should not exceed 10 µL, and for juvenile rats (P10) should not exceed 30 µL (Table 1).
- Follow step 1.1. The only exception is the injection volume in step 1.1.3.
- Restraining
- Using the dominant hand, hold the mouse/rat by the tail on a paper towel. The mouse/rat should be in the neutral prone position with the lateral aspect of the body facing you.
NOTE: Mice/rats may struggle due to restraint but should not elicit a specific pain response. If more than momentary pain responses are observed, administration of analgesics may be necessary; seek veterinary guidance. - Gently but firmly grasp the pelvic girdle between the non-dominant thumb and index finger. The iliac crest should be palpable and positioned in the center of the distal phalanx of each finger (Figure 1C).
NOTE: At this age, there is no concern about being bitten, so wrapping the paper towel over the mouse/rat is not required. It may be necessary to use a paper towel in P5-10 rats to keep the larger rat pups still. - Gently rotate the base of the tail to ensure proper alignment of the spine. The pelvis of the mouse/rat should be square so that the vertebral processes of the lumbar spine are perpendicular to the work surface.
- Swab the dorsal lumbar region of the mouse/rat with a 70% alcohol prep pad.
- Part the hair, if applicable, ~1-3 mm cranial to the iliac crest to help visualize the injection location. Visualization of the target area is easier in younger mice/rats. In hairless neonates, an indentation may be seen in the intervertebral space.
- Feel for or visualize the L4-L5 or L5-L6 intervertebral space, ~1-3 mm cranial to the iliac crest.
NOTE: The exact distance is variable depending on the size and age of the mouse/rat.
- Using the dominant hand, hold the mouse/rat by the tail on a paper towel. The mouse/rat should be in the neutral prone position with the lateral aspect of the body facing you.
- Injection
- Using the dominant hand, hold the center of the microliter syringe and position it at a 30-45-degree angle to the mouse/rat spine. With the bevel of the needle facing the head of the mouse/rat, puncture the skin approximately 1-3 mm caudle to the intervertebral space. Because of the angled orientation of the syringe, the point of insertion on the skin is slightly caudal to the intervertebral space.
NOTE: This distance may change with the age and size of the mouse/rat. - The remainder of steps are identical to steps 1.3.3-1.3.10 of mice >P21.
- Using the dominant hand, hold the center of the microliter syringe and position it at a 30-45-degree angle to the mouse/rat spine. With the bevel of the needle facing the head of the mouse/rat, puncture the skin approximately 1-3 mm caudle to the intervertebral space. Because of the angled orientation of the syringe, the point of insertion on the skin is slightly caudal to the intervertebral space.
3. Lumbar IT injection of rats >P21
NOTE: There are multiple IT injection procedures described in the literature, ranging from unanesthetized techniques to more extensive surgical approaches14,15. A procedure for direct injection using a minimally invasive technique is described using light anesthesia and a small skin incision. The use of anesthesia, such as isoflurane gas, can help with restraint, relax musculature, and prevent movement during the injection. Making a small incision in the skin over the injection site improves the accuracy of the injection by allowing visualization of the intervertebral space and eliminating the need to puncture through thick skin. Due to the incision, the use of anesthetics and analgesics is required. With practice, it is possible to perform lumbar IT injections in rats older than P21 without anesthesia or an incision at the user's discretion and pending institutional requirements15. Follow institutional guidelines and considerations regarding anesthesia, appropriate analgesics, and survival surgery for laboratory animals.
- Preparation
- Follow step 1.1. The only exceptions are the injection volume in step 1.1.3 and needle size in step 1.1.4. Injection volumes for rats can vary greatly by age and body size. See the Discussion section for more on injection volume at different ages (Table 1).
NOTE: A 27 G x 1" needle is recommended for adult rats, as smaller gauge needles may bend, and shorter needles may not adequately penetrate the intrathecal space. A longer (1.25-1.5") needle can be used if desired. - Anesthetize the rat using the preferred and approved anesthetic (following institutionally approved protocols).
NOTE: For the procedures described here, rats were anesthetized using isoflurane gas and a 1:1 mixture of lidocaine: bupivacaine was used as a local anesthetic. Body temperature should be maintained using an approved external heat source throughout the procedure and recovery. - Follow standard aseptic surgical site preparation for each animal. Briefly, shave the dorsal lumbar region and prep the skin with a surgical scrub, betadine, and 70% isopropyl alcohol.
- Follow step 1.1. The only exceptions are the injection volume in step 1.1.3 and needle size in step 1.1.4. Injection volumes for rats can vary greatly by age and body size. See the Discussion section for more on injection volume at different ages (Table 1).
- Surgical procedure
- Place the rat in the neutral prone position with the lateral aspect of the body facing you.
- Palpate the pelvic girdle, identifying the iliac crest and lumbosacral junction. In rats >P21, the vertebral processes can be easily identified.
- Using a scalpel, make a 1 cm incision over the L4-L5 or L5-L6 intervertebral space. The incision should only involve the skin and underlying fascia, leaving the muscle intact.
NOTE: It is helpful to use retractors to hold the skin open during the remainder of the procedure. - Gently but firmly grasp the pelvic girdle between the non-dominant thumb and index finger. The iliac crest should be palpable and positioned in the center of the distal phalanx of each finger (Figure 1D). Use mosquito forceps to probe along the midline of the back to locate the intervertebral space.
- Injection
- Using the dominant hand, hold the center of the microliter syringe and position it perpendicular to the rat spine at the L4-L5 or L5-L6 intervertebral space. Ensure that the plunger of the syringe can be depressed with the index finger without excessive movement of the hand. With the bevel of the needle facing the head of the rat, puncture the muscle with the needle over the intervertebral space.
- Advance the needle into the intervertebral space.
NOTE: The needle may have slight resistance as it passes between the vertebral processes, and the angle of the syringe may need to be adjusted slightly to pass through this gap. However, it should remain approximately 90 degrees, perpendicular to the spine. A pop will be felt as the needle enters the intrathecal space. Depending on anesthetic parameters, a tail or leg flick may or may not be noticeable. The needle should be tightly clamped between the vertebrae. - Using care not to move the syringe out of position, depress the plunger to deliver the solution within 30 s.
NOTE: As opposed to the injections in mice, it may not be possible to reduce the syringe angle in rats >P21 using this method. Larger volumes should be injected at a slower rate to prevent excessive pressure buildup. - Hold the syringe in place for 30-60 s after fully depressing the plunger to allow dissipation of the solution and prevent backflow.
- Smoothly withdraw the syringe from the rat. As the needle is withdrawn, rotate the syringe to prevent backflow.
NOTE: Use caution not to advance the needle when rotating the syringe, as this may cause physical damage to the spinal cord. - Close the skin incision using an approved method, such as suture or wound clips. For the rats used in this protocol, the skin was closed in a continuous intradermal or simple interrupted pattern using a 5-0 PDS II suture.
- Recover the rat from anesthesia.
- Follow steps 1.3.9-1.3.10 and all post-operative care guidelines.
Results
Although many factors can influence gene therapy vector transduction, histological staining of tissue remains the most accurate method for determining the success of lumbar intrathecal (IT) injections. Broad and even distribution of the gene therapy vector within the central nervous system (CNS) following injection is indicative of a successful procedure. Figure 2C represents a successful injection of a self-complementary AAV9-mediated gene therapy driving weak ubiquitous transgene expression under the JeT promoter at a dose of 1.3 × 1011 vg/mouse in neonatal (P1) mice, 4 months post-injection. RNAscope analysis, using a probe targeting the transgene delivered by the gene therapy, reveals broad distribution in the lumbar spinal cord, cervical spinal cord, and brain. A section of the liver and heart is included to highlight that, even with direct cerebrospinal fluid (CSF) injection, the gene therapy vector can still be distributed to peripheral tissues.
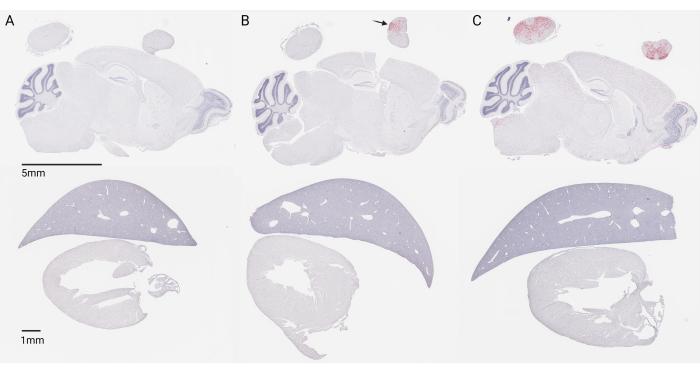
Figure 2: Stained 5-micron tissue sections of mouse CNS and peripheral tissue 4 months post-injection at P1. Red staining indicates transgene expression via RNAscope, and blue counterstaining of nuclei is done via hematoxylin. (A) Injection with a control formulation buffer. (B) Unsuccessful intraparenchymal injection with an AAV9 vector (scAAV9_JeT-hDDX3Xopt-SpA). (C) Successful lumbar IT injection with an AAV9 vector (scAAV9_JeT-hDDX3Xopt-SpA). The gene therapy vector used in (B) and (C) was given at a translationally relevant dose of 1.3E11 vg/mouse. Scale bar: top panel (5 mm); bottom panel (1 mm). Please click here to view a larger version of this figure.
Concentrated expression in the lumbar spinal cord paired with a lack of expression in the brain, as shown in Figure 2B, may indicate an intraparenchymal injection of the spinal cord and should be considered a failed injection. This happens when the needle is inserted too far into the spinal column, past the intrathecal space, and into the spinal cord. Additionally, very low or no expression in the spinal cord and brain (not shown) should also be considered a failed off-target injection, assuming that a vector and dose are being used where broad CNS distribution is expected. This may be due to not inserting the needle far enough or from being lateral to the midline.
The expression patterns observed from successful injections can differ due to the following eight factors as examples: (1) age at injection, (2) pre-existing immunity, (3) infusion rate, (4) gene therapy vector, (5) dose of gene therapy, (6) cell surface proteins, (7) tropism, and (8) if applicable, an appropriate promoter choice to drive transgene expression6. While expression patterns can differ, the broad, even, widespread distribution will be universal as long as the dose is sufficiently high with an effective vector, such as AAV9.
Major limitations of using histological analysis to confirm lumbar IT injection success are the extensive waiting times -- waiting until the end of a study, after necropsies are performed and tissue is collected -- and extensive resources needed if processing tissue from all mice in a large gene therapy study. Unfortunately, our experience has been that immediate and direct indicators of a positive or negative injection can be unreliable; however, the tail flick reflex as the needle enters the intrathecal space is a good indicator of successful positioning in real-time and likely indicates a successful injection. Do not confuse a twitch as the needle pierces the skin in an unanesthetized mouse/rat with a tail flick response to the needle entering the intrathecal space. The use of pharmacological agents such as NMDA, substance P, and lidocaine, either in training or mixed with the experimental injection solution, has been reported to provide a more immediate indication of injection success9,11,24. If considering these agents, it is important to evaluate their compatibility with the gene therapy vector.
Discussion
The lumbar IT injection is a quick and minimally invasive procedure that reliably delivers a gene therapy vector into the CSF for the treatment of CNS diseases5,6. The procedure is translationally relevant, and the protocol described here details how to perform this route of administration in mice and rats across all ages, from neonates to adults. It is important to define this protocol for mice and rats of all ages, along with providing supporting videos, to help investigators in the adoption of this method for gene therapy administration. Our laboratory's experience is that this protocol can be implemented consistently across multiple users and studies over time18,25,26,27,28,29,30.
There are important differences when performing the lumbar IT injection in younger mice/rats compared to older mice/rats, most notably the angle at which the needle is inserted into the spine and the recommended volume that is injected. Reported lumbar IT injection volumes vary considerably across studies and across species31. Consideration of the injection volume is important to avoid long-lasting elevations in intracranial pressure (ICP), which can compromise the flow of CSF and cerebral blood, cause discomfort, and lead to chronic neurological complications, including hydrocephalus, ischemia, cellular injury, and death32,33. ICP is determined by the volume of the CSF, cerebral blood, and CNS tissue, which cannot be directly correlated to body weight. At normal functioning, the ICP is autoregulated by many factors, including CSF volume, cerebral blood volume, respiration, body position, CSF production rate, and the rate of CSF drainage into the blood33,34. IT injection volumes should, therefore, be determined based on CSF properties (Table 1) instead of body weight25,27,28,30. Recommended volumes to inject at each age in each species are indicated in bold.
| IT Injection Volume | Adult CSF Values | |||||||
| P0-1 (µL) | P5-7 (µL) | P10 (µL) | >P21 (µL) | Total Volume (µL) | Rate of Production (µL/min) | Turnover (h) | Intracranial Pressure (mm Hg) | |
| Mice | 3 | 5 | 5-10 | 5-20 | 30-4025,30 | 0.32-0.3525,30 | 1.7-225,30 | 5.0 +/- 0.528 |
| Rats | 5 | 5-10 | 10-30 | 10-200 (20-75) | 15025 | 1.7-2.825 | 2-2.6625 | 8.6 +/- 1.7,27 |
Table 1: Summary of lumbar IT injection volumes for mice and rats at different ages. Bold values are recommended and have been delivered safely. Maximum possible volumes have not been formally evaluated. Additional information about known CSF parameters -- total volume, rate of production, turnover, and intracranial pressure -- in mice and rats is included for reference.
There is a lack of knowledge across the field regarding the upper threshold for one-time bolus IT injection volumes. In adult humans, rats, and mice where CSF volume is known, a 30% increase in total CSF volume does not seem to cause chronic injury or maladies31,33,35,36. The lack of known CSF volumes in juvenile or neonatal mice makes a similar extrapolation impossible. Some groups are beginning to look at CSF volume and production in younger animals37. Until additional investigations in these areas are substantiated, injection volume will continue to be subject to investigator-reported values.
Mice and rats, especially when treated at younger ages or with high injection volumes, may elicit flexing of muscles, extension of limbs, rapid breathing, or temporary hindlimb paralysis that should self-resolve within a few minutes. In extreme cases, acute ICP elevations can cause cardiovascular and respiratory abnormalities, which can be deadly32,33. If any post-procedural abnormalities persist after 24 h, the mice/rats should be removed from the study and humanely euthanized. Persistent hindlimb paralysis can occur if the needle is inserted too far, impacting the spinal cord. This can be due to a common mistake while performing the lumbar IT injection: movement of the syringe after the tail flick while depressing the plunger. Syringe and needle movement should be avoided. If the correct position cannot be acquired with the initial puncture, a second attempt at the same location can be made. If the second attempt is also unsuccessful, try altering the needle position to target the next intervertebral space. Note that multiple needle sticks may lead to leakage of a subsequently successful injection.
Becoming proficient at the lumbar IT injection can take time. To practice the injections as a terminal procedure, follow the above protocol using an approved dye solution, such as Evans blue or 0.2 microns filtered McCormick green dye (Figure 3) or with the pharmacological agents addressed in the Representative Results section. Using dye is recommended to troubleshoot and master the injection because it is easy to tell if an injection was a success or failure within 1 min. Practice with dye is for non-survival procedures only, as animals can develop a reaction to the dye when directly administered to the CNS. This reaction can occur within a minute of a successful injection and is characterized by rapid itching and squirming movements. The animals should be immediately euthanized once this reaction is observed to minimize discomfort. After a successful practice dye injection, dye will stay localized in the spine (no dye in nearby peripheral tissue), and move up the spinal column to the cerebellum, cerebrum, and olfactory bulbs. At P1, the skin is transparent enough that the dye can be seen moving down the spinal column in the tail. If the dye does not reach the brain within a few minutes, the injection fails.

Figure 3: McCormick green dye in the brain after successful practice lumbar IT injections. All brains are from P21 mice injected with 5 µL dye and are shown in the ventral view. Please click here to view a larger version of this figure.
For additional information on relevant associated parameters for preclinical trial design, like viral titer and doses, see previously published reviews3,6,31.
Disclosures
The authors declare no conflicts of interest.
Acknowledgements
We would like to thank the UT Southwestern AAV Viral Vector Core Facility for manufacturing the AAV9 vector and Yuhui Hu, Research Scientist in the Gray Lab, for processing and staining the tissue presented in Figure 2.
Materials
| Name | Company | Catalog Number | Comments |
| 0.2 micron filter | Electron Microscopy Sciences | 67005 | Used to filter dye solution |
| 0.5 to 10 µL Pipette | Eppendorf | TI13690026 | Used to measure injection solution |
| 1.5 mL Microtube | Eppendorf | 22364111 | Used to store injection solutions |
| 10 µL Syringe | Hamilton | 7635-01 | Injection volume should not be less than 10% of syringe capacity |
| 10 to 100 µL Pipette | Eppendorf | TI13690029 | Used to measure injection solution |
| 10µl Pipette Tips | USA Scientific Inc | 11203810 | Used to measure injection solution |
| 100 µL Syringe | Hamilton | 7638-01 | For rat >21 only. Injection volume should not be less than 10% of syringe capacity |
| 100 µL Pipette Tips | USA Scientific Inc | 11231840 | Used to measure injection solution |
| 25 µL Syringe | Hamilton | 7636-01 | Ideal for 5-10 µL injections. Injection volume should not be less than 10% of syringe capacity |
| 27 Gauge Needle(s) | Hamilton | 7803-01 | For rat >21 only. 27 gauge, Small Hub RN Needle, 1 in, point style 4 at 12°, 6/PK |
| 30 Gauge Needle(s) | Hamilton | 7803-17 | 30 gauge, Small Hub RN Needle, 0.5 in, point style 4 at 12°, 6/PK |
| 50 µL Syringe | Hamilton | 7637-01 | For rat >21 only. Injection volume should not be less than 10% of syringe capacity |
| 70% Ethanol | Pharmco | 111000140 | Used to sanitize workspace and equipment |
| 70% Isopropyl Alcohol Prep Pads | PDI | B60307 | Used to prepare injection site |
| Analgesic | For rat >21 only. | ||
| Anesthetic (Isoflurane) | Piramal Critical Care | 66794001725 | For rat >21 only. |
| Betadine | Purdue Products | 6906606 | For rat >21 only. Used for skin prep |
| Control Solution | Injection solution | ||
| Dye Solution (green) | McCormick | For practice, non-survival only | |
| Gloves | Kimberly-Clark | 19-149-863B | PPE |
| Ice bucket with ice | Fisher Scientific | 03-395-150 | Maintain viral vector solution on ice |
| Mosquito Forceps (curved or straight) | Fine Science Tools | 13009-12 | For rat >21 only. Used to palpate intervertebral space. |
| Needle Holders | Fine Science Tools | 12002-12 | For rat >21 only. Used for skin closure with suture |
| Paper Towel | Berkshire | 18-998-123 | Used to restrain adult mice during injection |
| Parafilm | StatLab | PM996 | Used to draw solution into syringe |
| Retractors | Stoelting | 52124P | For rat >21 only. Used to hold skin incision open |
| Scalpel Blade | Fine Science Tools | 10015-00 | For rat >21 only. Used for incision |
| Scalpel Blade Handle | Fine Science Tools | 10003-12 | For rat >21 only. Used for incision |
| Sterile Syringe | Fisher Scientific | 14-955-459 | Used to filter dye solution |
| Surgical Scrub (Skin Prep) | Medline Industries Inc. | MDS098720 | For rat >21 only. Used for skin prep |
| Suture or Wound Clips | Stoelting | 50483 | For rat >21 only. Used for skin closure. |
| Syringe / Needle Cleaning Solution | Hamilton | 18311 | Can use alternative cleaning solution |
| Thumb Forceps | Fine Science Tools | 11019-12 | For rat >21 only. Used throughout surgical approach and closure |
| Vector Solution | Injection solution |
References
- Deverman, B. E., Ravina, B. M., Bankiewicz, K. S., Paul, S. M., Sah, D. W. Y. Gene therapy for neurological disorders: Progress and prospects. Nat Rev Drug Discov. 17 (9), 641-659 (2018).
- Pupo, A., et al. AAV vectors: The Rubik's cube of human gene therapy. Mol Ther. 30 (12), 3515-3541 (2022).
- Ling, Q., Herstine, J. A., Bradbury, A., Gray, S. J. AAV-based in vivo gene therapy for neurological disorders. Nat Rev Drug Discov. 22 (10), 789-806 (2023).
- Lykken, E. A., Shyng, C., Edwards, R. J., Rozenberg, A., Gray, S. J. Recent progress and considerations for AAV gene therapies targeting the central nervous system. J Neurodev Disord. 10 (1), 16 (2018).
- Hocquemiller, M., Giersch, L., Audrain, M., Parker, S., Cartier, N. Adeno-associated virus-based gene therapy for CNS diseases. Hum Gene Ther. 27 (7), 478-496 (2016).
- Chen, X., et al. Biodistribution of adeno-associated virus gene therapy following cerebrospinal fluid-directed administration. Hum Gene Ther. 34 (3-4), 94-111 (2023).
- Corning, J. L. Spinal anesthesia and local medication of the cord. NY Med. J. , 483-485 (1885).
- Hylden, J. L., Wilcox, G. L. Intrathecal morphine in mice: A new technique. Eur J Pharmacol. 67 (2-3), 313-316 (1980).
- Fairbanks, C. A. Spinal delivery of analgesics in experimental models of pain and analgesia. Adv Drug Deliv Rev. 55 (8), 1007-1041 (2003).
- Choi, S. E., et al. High-frequency ultrasound-guided intrathecal injections in a young mouse model: Targeting the central nervous system in drug delivery. J Neurosci Methods. 386, 109778 (2023).
- Li, D., Li, Y., Tian, Y., Xu, Z., Guo, Y. Direct intrathecal injection of recombinant adeno-associated viruses in adult mice. J Vis Exp. 144, e58565 (2019).
- Njoo, C., Heinl, C., Kuner, R. In vivo SiRNA transfection and gene knockdown in spinal cord via rapid noninvasive lumbar intrathecal injections in mice. J Vis Exp. 85, e51229 (2014).
- Vulchanova, L., et al. Differential adeno-associated virus-mediated gene transfer to sensory neurons following intrathecal delivery by direct lumbar puncture. Mol Pain. 6, 31 (2010).
- Yaksh, T. L., Rudy, T. A. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 17 (6), 1031-1036 (1976).
- Mestre, C., Pelissier, T., Fialip, J., Wilcox, G., Eschalier, A. A method to perform direct transcutaneous intrathecal injection in rats. J Pharmacol Toxicol Methods. 32 (4), 197-200 (1994).
- Donsante, A., Rasmussen, S. A., Fridovich-Keil, J. L. Intrathecal vector delivery in juvenile rats via lumbar cistern injection. J Vis Exp. 205, e66463 (2024).
- Lu, X., Jiang, Y. H. Intrathecal injection of newborn mouse for genome editing and drug delivery. J Vis Exp. 205, e65761 (2024).
- Chen, X., et al. Intrathecal AAV9/AP4M1 gene therapy for hereditary spastic paraplegia 50 shows safety and efficacy in preclinical studies. J Clin Invest. 133 (10), JCI164575 (2023).
- Deschenes, N. M., et al. Biochemical correction of GM2 ganglioside accumulation in AB-variant GM2 gangliosidosis. Int J Mol Sci. 24 (11), ijms24119217 (2023).
- Hwang, S. M., Rahman, M. M., Go, E. J., Kim, Y. H., Park, C. K. Specific transcription factors Ascl1 and Lhx6 attenuate diabetic neuropathic pain by modulating spinal neuroinflammation and microglial activation in mice. Biomed Pharmacother. 173, 116392 (2024).
- Kagiava, A., et al. Gene replacement therapy in two Golgi-retained CMT1X mutants before and after the onset of demyelinating neuropathy. Mol Ther Methods Clin Dev. 30, 377-393 (2023).
- Wong, H., et al. CNS-dominant human FMRP isoform rescues seizures, fear, and sleep abnormalities in Fmr1-KO mice. JCI Insight. 8 (11), 169650 (2023).
- Laoharawee, K., et al. Prevention of neurocognitive deficiency in mucopolysaccharidosis type ii mice by central nervous system-directed, AAV9-mediated iduronate sulfatase gene transfer. Hum Gene Ther. 28 (8), 626-638 (2017).
- Aanonsen, L. M., Wilcox, G. L. Phencyclidine selectively blocks a spinal action of N-methyl-D-aspartate in mice. Neurosci Lett. 67 (2), 191-197 (1986).
- Bailey, R. M., Armao, D., Nagabhushan Kalburgi, S., Gray, S. J. Development of intrathecal AAV9 Gene therapy for giant axonal neuropathy. Mol Ther Methods Clin Dev. 9, 160-171 (2018).
- Bailey, R. M., Rozenberg, A., Gray, S. J. Comparison of high-dose intracisterna magna and lumbar puncture intrathecal delivery of AAV9 in mice to treat neuropathies. Brain Res. 1739, 146832 (2020).
- Chen, X., et al. AAV9/MFSD8 gene therapy is effective in preclinical models of neuronal ceroid lipofuscinosis type 7 disease. J Clin Invest. 132 (5), JCI146286 (2022).
- Karumuthil-Melethil, S., et al. Intrathecal administration of AAV/GALC vectors in 10-11-day-old twitcher mice improves survival and is enhanced by bone marrow transplant. J Neurosci Res. 94 (11), 1138-1151 (2016).
- Ling, Q., Rioux, M., Hu, Y., Lee, M., Gray, S. J. Adeno-associated viral vector serotype 9-based gene replacement therapy for SURF1-related Leigh syndrome. Mol Ther Methods Clin Dev. 23, 158-168 (2021).
- Sinnett, S. E., Boyle, E., Lyons, C., Gray, S. J. Engineered microRNA-based regulatory element permits safe high-dose miniMECP2 gene therapy in Rett mice. Brain. 144 (10), 3005-3019 (2021).
- Rahman, M. M., Lee, J. Y., Kim, Y. H., Park, C. K. Epidural and Intrathecal Drug Delivery in Rats and Mice for Experimental Research: Fundamental Concepts, Techniques, Precaution, and Application. Biomedicines. 11 (5), 11051413 (2023).
- Allen, C. H., Ward, J. D. An evidence-based approach to management of increased intracranial pressure. Crit Care Clin. 14 (3), 485-495 (1998).
- Belov, V., et al. Large-volume intrathecal administrations: Impact on CSF pressure and safety implications. Front Neurosci. 15, 604197 (2021).
- Moazen, M., et al. Intracranial pressure changes during mouse development. J Biomech. 49 (1), 123-126 (2016).
- Rieselbach, R. E., Di Chiro, G., Freireich, E. J., Rall, D. P. Subarachnoid distribution of drugs after lumbar injection. N Engl J Med. 267, 1273-1278 (1962).
- Pardridge, W. M. CSF, blood-brain barrier, and brain drug delivery. Expert Opin Drug Deliv. 13 (7), 963-975 (2016).
- Ghersi-Egea, J. F., Babikian, A., Blondel, S., Strazielle, N. Changes in the cerebrospinal fluid circulatory system of the developing rat: quantitative volumetric analysis and effect on blood-CSF permeability interpretation. Fluids Barriers CNS. 12, 8 (2015).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved