Method Article
A Rapid Method to Confine and Safely Handle Bees in the Field
In This Article
Summary
We demonstrate a tested method for safely handling field-collected bees. This method allows swift manipulation, identification, genetic sampling, and confirmation of plant-insect interactions via pollen collected while sampling. Easily adaptable, this approach offers a cost-effective, non-lethal means to study rare insect groups.
Abstract
Improving understanding of the basic biology and ecology of many insect pollinators, particularly specialist or rare taxa, is a priority for many researchers. As such, there is often a need to temporarily confine field-collected organisms in a non-injurious manner in order to gain information or support additional studies. This protocol represents a thoroughly tested, quick, and inexpensive field method for safely handling bees of conservation concern that can easily be tailored toward specific project needs, including organism identification, pollen removal, marking, and/or collection of non-lethal tissue samples for genetic analysis. This methodology can serve as an additional option in the researcher's toolbox to use when certain scenarios arise. It is anticipated that this methodology can be adapted for use with other insect species as well as used by individuals of varying experience and skill levels. It can be of great value to researchers studying specialist bees or conducting host-specific studies. The data collection made possible by this protocol will be invaluable to help researchers address critical data gaps for many pollinator species, plant-pollinator network structures, and pollinator conservation and management initiatives.
Introduction
A growing body of evidence supports wild bee and other pollinator population declines and accompanying pollinator community changes1,2,3,4. Continued losses threaten the very service of insect pollination vital to biodiversity maintenance, ecosystem function, and agricultural production5. Moreover, for many wild bees, especially rare species, significant knowledge gaps exist that can hinder appropriate management and conservation actions6,7.
To help address these data deficiencies, researchers have developed a variety of methods to study insect pollinators, associated habitat usage, and their floral preferences. While pan traps, blue-vane traps, malaise traps, emergence traps, and direct collection by hand netting are commonly utilized, many of these methods have significant drawbacks8,9,10,11. Commonly employed methods to identify the pollinator can result in organism mortality, regardless of whether the specimen must be identified in a lab setting (e.g., using a microscope). Mortality can be justifiable and necessary for many insect studies. However, when working with imperiled, rare, or understudied insects whose population statuses are limited or uncertain, researchers must mitigate organism mortality, injury, or stress to reduce the likelihood of negatively impacting these insect populations. Therefore, when working with at-risk species or species that can be easily identified by their key distinguishing features, less destructive sampling approaches should be taken if possible.
Non-lethal methods that have been proposed for the collection of genetic material from bees include collection of feces, exuviae12, and wing tips13. However, utilizing these methods on bees collected in the field may be untenable due to the time required and/or potential impact on wings, negatively affecting flight and other behaviors. Partial antennae removal has been shown to not compromise the survivorship of sampled euglossine bees14. Likewise, sampling of the terminal portion of the tarsus of the mid-leg did not significantly reduce Bombus terrestris worker survivorship15. An additional non-lethal sampling method involves collecting protein residues by temporarily immersing bees in a buffer solution and then subsequently releasing them16. Survival analysis showed that there were no significant differences between buffer-rinsed and unrinsed bees. There are limitations to each technique, which should be considered when addressing specific research questions and overall project goals.
Accurate taxonomic identification of organisms is critical for effective research. For many insect pollinator taxa, however, it is extremely contingent on the species of interest and the knowledge and experience level of the researcher or observer. While many bee species can be identified in the field, having evidence to support the observation can be critical. While most pollinator studies typically collect and retain individuals as evidence, the use of photos and videos, as well as three-dimensional videography using virtual reality can be utilized as a proxy to distinguish certain species without the sacrifice of the individuals being observed17. Differentiation between some species may require special attention and photographs of specific morphological features; in these situations, the organisms must be able to be manipulated and confined to a unique position such that the complex distinguishing characters can be reliably photographed.
Temporarily confining bees for identification can be done in several ways, including cooling the specimen and/or using carbon dioxide to slow bees18,19. However, these methods may alter behavior, resulting in treated bees being slower to regain activity, thereby possibly affecting foraging, organism fitness, or increasing the risk of predation20,21,22. Additionally, such techniques ultimately increase the time that organisms are confined and handled. This, in turn, increases organism stress and field processing time. Safer and more efficient methodologies would, therefore, be highly desirable.
A number of studies have used the pollen collected from bees or other sources to better understand foraging preferences, construct plant-pollinator interaction networks, identify environmental contamination (e.g., pesticide residues), and evaluate nutritional ecology23,24,25,26,27,28,29. Many bees will self-groom when confined in a container. Therefore, non-lethal methods of sampling for pollen have been utilized30 (e.g., microcentrifuge tubes). However, in cases where self-grooming does not take place, using a more tactile container, such as the resealable plastic bags used in this protocol, allows for gentle pressure to be applied to specific body parts so that the pollen comes in contact with the plastic bag, leading to a higher likelihood of getting a pollen sample than the use of traditional hard containers.
Here, we present a protocol that has been well-tested on three at-risk bee taxa. While labor intensive, it allows for comprehensive data collection from insect pollinators while minimizing the threat of mortality to the individual organisms. The overall goal of using this methodology is to provide a safe and effective means to capture, identify, and safely release insects. An added advantage of this protocol is that it overcomes many of the limitations of traditional insect collecting. It provides an easy way to mark individuals, collect non-lethal genetic material, and collect pollen samples, all while minimizing handling time and stress on the organism. While traditional insect collecting methods have many benefits31, to help overcome some of their limitations, we established an alternative so that insects can be confined for identification before a quick and safe release. Depending upon project goals, additional steps can also be taken while the bee is confined to collect other important data.
Protocol
1. Field collection preparation
- Confirm project goals (e.g., organism identification, genetic tissue sampling, etc.).
- Review the Table of Materials and gather all relevant items specific to the project goals.
- Ensure all digital equipment (e.g., smartphone, camera, handheld global positioning system [GPS]) is fully charged and that spare batteries are charged and packed.
2. Capturing and securing organism
- Record site parameters of interest upon arriving in the field, including date, start time, field site/location, and any other relevant information (e.g., weather conditions, dominant ground cover plants, plants in bloom, etc.) that may be needed (Figure 1).
- Capture an individual bee of interest using the appropriate netting technique. Use hand netting via an aerial insect net or sweep net based on the focal species.
NOTE: Other capture techniques, such as collecting via vial/centrifuge tube, could also be used for insect capture. - Visually observe the specimen through the net bag to determine if it resembles the taxon of interest. If not, safely release the specimen and continue surveying.
- If the specimen appears to be the focal species, secure the specimen within the net bag so that it cannot escape (e.g., by overlapping the top of the net bag over the frame, twisting/confining the neck of the net bag, or otherwise closing off any potential exits).
- Gather the resealable sample bag and open the sample bag.
- Ensure that the bee of interest is near the tip of the net bag.
- With one hand, grasp the net bag immediately below the specimen. Hold the net bag such that the tip (where the insect is confined) is oriented upwards and the net opening (i.e., hoop) hangs below.
NOTE: Most insects are phototrophic and, when confined, generally fly/crawl towards light. - Using the other hand (i.e., the hand not holding the net bag), guide the resealable sample bag in the net opening and through the net bag until one reaches the hand immediately below the specimen.
- Carefully release the grip of the hand, confining the specimen just enough to enable the hand holding the resealable sample bag to move into the confined area with the specimen. Be mindful of the specimen's location within the confined area to reduce the likelihood of being stung, harming the specimen, and escaping.
- Manipulate the resealable sample bag to open wide enough to allow the insect specimen to enter. Do this by applying pressure on either side of the seal or twisting the bag with the thumb and middle finger below the seal.
- Position the resealable sample bag opening above the specimen and gently maneuver the insect into the bag. As mentioned previously, since most insects are phototrophic, orient the hand containing the resealable sample bag towards the sun/sky, thereby facilitating specimen movement into the bag.
- Once the specimen is inside, firmly seal the resealable sample bag.
- Remove the resealable sample bag containing the specimen from the insect net.
NOTE: As insects can quickly and lethally overheat in sealed bags, keep the specimen out of direct sun exposure, ideally in a shaded location or insulated container until processing, and limit processing time.
3. Identify the organism
- Closely inspect the specimen to confirm it is a taxon of interest. If it is a different species, safely release it and continue surveying.
NOTE: To avoid harm to the specimen, never apply direct pressure to the insect while it is inside the bag. Specimens can be immobilized by applying gentle pressure to the plastic or by stretching the perimeter of the bag to make the bag taut around the specimen, thereby limiting movement. - If species identity can easily and accurately be confirmed visually, take a photo voucher (Figure 2). Record any additional necessary information about the specimen (e.g., time of capture, specific GPS location, plant visited, unique markings, size or coloration observation, behavior before capture, etc.).
- If specific physical features need to be inspected to confirm identity, take detailed macro photographs highlighting those key features through the resealable sample bag (Figure 2).
- If photos of sufficient quality for feature discernment are not attainable through the sample bag, expose the specimen's body part(s) of interest for close inspection by cutting one of the two non-sealed corner tips of the sample bag (i.e., the corners that are seamed together or not resealable). For example, cut a small hole to expose only the head, abdomen, or leg (Figure 3A-C). For this, manipulate the specimen such that the body part of interest moves first toward the cut/corner hole.
NOTE: The size and position of the hole cut in the bag and the orientation of the insect may need to be altered to obtain the necessary photograph. - After identification has taken place, skip to the relevant sections for subsequent and desired methods. See section 4 for antennal segment removal technique, section 5 for insect marking, and/or section 6 for obtaining pollen samples.
4. Obtaining non-lethal genetic samples from antennae
- Use scissors to diagonally cut one of the two non-sealed corners (i.e., the corners that are seamed together or not resealable) of the resealable sample bag. Ensure that the cut made is minimally larger than the width of the bee head (Figure 4).
- Manipulate the specimen so it moves headfirst toward the cut/corner hole.
NOTE: This step can be adapted to collect other tissue samples for genetic analysis (e.g., whole leg, partial leg). Accordingly, the size and position of the hole cut in the bag and the orientation of the insect may need to be altered to obtain the necessary sample. - Once the bee's head is protruding from the bag, gently apply pressure to the surrounding plastic to make it taut around the insect, restricting movement (Figure 3A).
- If the hole is too large, roll the bag over itself to further restrict the hole opening and secure the specimen. If unsure of the appropriate hole size, perform steps 4.2 and 4.3 inside an insect net or flight cage to ensure the specimen does not fully escape. Use an additional bag if the original corner cut is too large.
- Position the bag such that the insect head is directly over the collection container (e.g., microcentrifuge tube/vial containing buffer solution/ethanol) and that the container for the genetic sample is appropriately marked with the unique specimen ID corresponding to all other specimen data (Figure 3D).
- Using clean and sterilized dissecting scissors, cut a portion of one antennal segment. Visually inspect the container to confirm that the sample is inside the container.
NOTE: When cutting, it is helpful to work over a clean, sterilized, light-colored substrate (e.g., Kimwipe). This ensures that if the sample does not fall into the sample collection container, it can easily be retrieved with forceps with minimal risk of contamination. - Secure the tissue sample collection container lid and rotate the container so that the sample is suspended within the solution (e.g., buffer solution/ethanol).
- Place the tissue sample collection container (with the antennal sample) in a secure container, ideally in a cool, shaded place protected from direct sunlight and/or extreme temperatures, such as a field cooler.
- Safely release the specimen near the original point of capture.
NOTE: Specimen could also be marked (see section 5) prior to release to readily identify it as having been sampled if it is resighted/recaught.
5. Marking the organism
- With the specimen in the resealable sample bag, cut a small hole in the middle of the sample bag.
NOTE: This hole is in addition to the hole created in section 4. The hole should be no larger than the area of the insect's thorax. The position of where the hole should be cut can vary based on the size of the insect and the desired marking area. - Applying gentle pressure to the plastic on both sides of the specimen, maneuver the insect such that the thorax is directly under the hole (i.e., that the top of the thorax is exposed through the bag). Continue with gentle pressure to ensure the specimen remains in place (Figure 5A).
NOTE: Other marking areas may be better for certain insects (Figure 5B). Some users find it more helpful to make the existing hole (from section 4) larger and grab the bee by holding its thorax as it emerges (Figure 5C). This approach may increase the chance of getting stung. Additionally, honey bee queen marking devices can be modified to confine and mark bees if the user finds that easier. However, this method requires transfer to a different device and could contaminate pollen samples. - Using a paint marking pen (or other marking material deemed appropriate for the taxon of interest), mark the specimen according to the predetermined project-specific methodology.
NOTE: Marking methods will differ based on the goals and may be simple, indicating the individual was captured, or complex, allowing for identification of individuals (e.g., using unique color coding or patterning) (Figure 5C). - Hold the specimen in place until the applied mark is adequately dry.
- Photograph the marked individual for confirmation of unique coloration and color position.
NOTE: Recapture individuals can easily and quickly be photographed directly through the resealable sample bag (Figure 5D). - Safely release the specimen near the original point of capture.
6. Pollen sample collection
- With the specimen in the resealable sample bag, carefully inspect it for any visible pollen.
NOTE: As pollen type and quantity vary tremendously, sometimes pollen is not visible on the specimen with the naked eye. If the previous steps have already been completed, it is possible that pollen remnants from the specimen are already in the bag. - If pollen is visible on the specimen, confine the specimen's movement by applying gentle pressure to the plastic on both sides of it.
- Using a finger, gently rub or push the plastic against setae or pollen-containing body part to facilitate the removal of pollen.
- If pollen is not visible on the specimen, maximize the contact between the specimen and the plastic to see if any small pollen remnants are removed from the integument.
- Visibly confirm that pollen is in the resealable sample bag, if possible (Figure 4).
- Safely release the specimen near the original point of capture.
- Firmly seal the resealable sample bag containing the pollen sample.
NOTE: If a hole was cut in the resealable sample bag, it should be placed within another resealable sample bag to avoid contamination or loss of pollen. - Label the resealable sample bag with a unique specimen ID corresponding to the individual insect and other data (e.g., insect species ID, date, location, time, sex, floral visitation record, etc.).
- Place the resealable sample bag with the pollen sample in a secure container, ideally in a cooler place, to protect it from direct sunlight and/or extreme temperatures.
NOTE: If appropriate, follow project-specific protocols for field-based pollen preservation (e.g., genetic analysis, pollen morphology).
Results
This methodology has been used for three at-risk bee species (Osmia calaminthae, Caupolicana floridana, and C. electa) in the southeastern United States. To date, hundreds of bees and wasps have safely been collected and released. No bees died while using this methodology; those designated as voucher specimens and kept as a new location record with the appropriate managing agency were appropriately sacrificed after data collection. Table 1 shows different morphological features evaluated as well as other quantifiable data that can be collected using this protocol14,32,33,34,35,36.
Figure 1: Example data sheet showing data that could be collected while in the field. The specific data collected will vary based on project goals. Please click here to view a larger version of this figure.

Figure 2: Photos to serve as vouchers. Taking photos to serve as vouchers of the occurrence is essential for reporting purposes. Photos of distinct identifying features are necessary when multiple species share similar characteristics. This Anthidium maculifrons found in Florida can be distinguished from others in the genus based upon the yellow on its scape and head. Please click here to view a larger version of this figure.
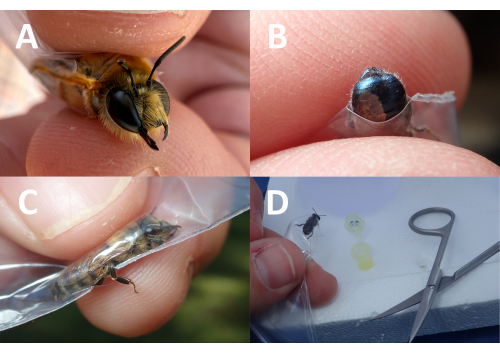
Figure 3: Placing the hole in the resealable sample bag. The placement of the hole in the resealable sample bag can be altered to get specific body parts of interest exposed for photographs or genetic samples. In this composite photo, the (A) bee's head, (B) abdomen, and (C) leg are exposed to the photograph. Once the bee is confined and cannot move, it often rests and can be positioned to get a macrophotograph. (D) A genetic sample can also be taken when the bee is in these positions. Please click here to view a larger version of this figure.

Figure 4: Collection bag with bee showing one corner diagonally cut. If wanting to closely observe the head of the bee, the cut on the corner of the bag will vary in size based on the head size of the bee. Pollen and even nectar secretions may be found in the bag for future pollen identification. Please click here to view a larger version of this figure.

Figure 5: Images of resealable sample bag with bees. To avoid getting stung while marking the bee, a hole can be made in the bag, and the (A) thorax can be positioned under the hole. (B) Depending upon the size of the bee, it can also be marked on the abdomen. (C) Alternatively, the bee can also be released from the corner hole and compressed at the thorax for marking. This technique can increase the chance of getting stung but seems to minimize pen smearing. Unique coloring/numbering can be used to differentiate between individuals. (D) Future recaptured specimens can quickly and easily be photographed through the resealable sample bag and released. Please click here to view a larger version of this figure.
Table 1: Morphological features evaluated using this protocol. Samples can also be manipulated to observe and document numerous traits not represented in this table (e.g., tergite/sternite shape, overall length, weight, number of teeth, wing venation, intertegular distance, etc.). Please click here to download this Table.
Discussion
This protocol outlines a field method for safely handling and inspecting rare bees with the end goal of obtaining desired non-lethal sample or voucher information and safely releasing focal individuals back into the wild at the original point of capture. The benefits of this protocol over other collection methods, such as the use of vials, are that the specimen can be safely confined to allow for close examination of key features and confident identification, limiting harm to both the insect and investigator. Conversely, as is the case with other methodologies18,19, this protocol does not require the specimen to be anesthetized; it can be sampled and released quickly with minimal handling. Resealable sample bags are low-cost, easy to acquire, lightweight, extremely portable, and recyclable, making them a great alternative to centrifuge tubes. As they lack the rigidity of some alternatives (e.g., falcon tubes or other hard containers), it is important to take extra care when handling live insect specimens. If an entire specimen is to be taken as a voucher, placing it in a sturdy enclosure will reduce potential damage to the specimen.
It is beneficial for researchers using this method to have experience with handling bees and/or other insects because applying too much pressure on the specimens while they are in the bag could result in injury or mortality. To get more experience handling bees, novice researchers should practice this protocol using more common species (e.g., honey bees). Practice will help minimize injury or mortality to the insect. It is important to note that depending upon the focal taxon, there may be limitations to this methodology. The reduced size of specific taxa may require the use of more costly and specialized macro photography equipment and/or the use of field microscopes as their features may not be able to be isolated and photographed with the materials listed in this procedure, the smaller the target, the harder it can be to get adequate images37. Additionally, in instances where inaccessible body parts are required (e.g., tongue, genitalia, etc.), other methods for identification may be warranted. Genitalia is among the most informative diagnostic traits for insects, which can be highly variable between species and somewhat stable within them38,39. In this case, lethal methods, such as dissection, may be necessary. However, for hard-to-identify species, the use of small, non-lethal genetic samples can be used for identification after field collection40, and the methodology described here can be used to collect such samples. Statistical modeling is also being developed to help associate imaging and DNA sequencing for insect identification41.
Another limitation of the methodology presented here concerns the probability of getting stung when performing this protocol, especially when having a hole cut in the bag. This protocol, however, minimizes the likelihood of getting stung; the authors have only rarely been stung through specimen bags while handling specimens. It should also be noted that some species of bees, beetles, and wasps have been able to cut the bags using their mandibles, so care should be taken when determining if this approach will work for the taxa of interest and, in these instances, thicker plastic bags or other methodologies would be recommended. In all instances, users should minimize using one-time-use plastics and recycle when possible.
The focal taxon for the development of this protocol was the blue calamintha bee, Osmia calaminthae (Hymenoptera: Megachilidae), which measures approximately 10-11 mm in size32. Since developing this method, the authors have employed it on a variety of other hymenopterans of various sizes, including larger Bombus species (Hymnenoptera: Apidae) and Caupolicana species, C. electa and C. floridana (Hymenoptera: Colletidae). Caupolicana electa can vary from 18-23 mm, while C. floridana can vary from 16-18 mm33. To help minimize any negative impacts to at-risk, imperiled or listed species, it is recommended to try it on closely related and/or common surrogates first to help gain experience and build proficiency. The exoskeleton of bees and other insects can vary, and less robust specimens should be treated with care. In situations where smaller or softer bodies of insects are being studied, this methodology may not be sufficient. Users must determine which parts of this methodology will be appropriate for their focal taxon.
Beyond the primary goal of confining field-collected organisms for identification, this protocol can be modified to perform various research-related tasks for which bees need to be safely confined. For example, organisms can be weighed in the field while in the resealable sample bags. Researchers can also take various measurements of specimens using calipers while the insect is constrained. For example, the estimation of the homing ability of bees can be done using body size42; our methodology could help acquire data that would facilitate such estimation. Likewise, instead of using calipers, researchers can place and photograph a ruler/scale bar and/or color card directly behind the specimen to measure key morphological features when processing images later. Future applications of this method could leverage advancements in artificial intelligence and machine learning. Identification, both in the field and in the lab, could be streamlined using smart devices, thereby minimizing handling time and stress on specimens.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors wish to thank Ivone de Bem Oliveira, Jon Elmquist, Emily Khazan, Nancy Kimmel, and Kristin Rossetti for reviewing this manuscript. This research was funded through a grant from the U.S. Fish and Wildlife Service administered by the Florida Fish & Wildlife Conservation Commission (Agreement No. 19008) and funds from the Florida Biodiversity Foundation.
Materials
| Name | Company | Catalog Number | Comments |
| 30x 60x illuminated jewelers eye loupe magnifier | JARLINK | Hand lens (if necessary) for observing diagnostic characteristics | |
| Aerial hand net | |||
| Bleech in wash bottle | Only needed for non-lethal genetic sampling | ||
| Blunt-tip kids scissors | Fiskar | Blunt-tip scissors are beneficial because they can safely be kept in pockets | |
| Ethanol in wash bottle | Only needed for non-lethal genetic sampling | ||
| FD-1 flash diffuser | Olympus | Flash Diffuser to illuminate specimen while taking voucher photos | |
| Field clipboard | |||
| Field cooler | |||
| Fine forceps | |||
| Fine point oil-based paint marker set | Sharpie | Pens to mark bees | |
| Kimwipes | Kimtech | ||
| Microcentrifuge tubes | Only needed for non-lethal genetic sampling | ||
| Resealable sample bag | Amazon | Dependent on specimen of interest. We prefer 50.8 mm x 76.2 mm or 50.8 mm x 50.8 mm - Edvision 2" x 3" Plastic Bags, 200 Count 2 Mil Transparent Resealable Zipper Poly Bags, Reclosable Storage Bags for Jewelry Supplies, Beads, Screws, Small Items - Soft 'N Style 500 Count Resealable Zipper Poly Bags, 2 by 2-Inch, 50mm by 50mm, Clear | |
| Stainless steel iris dissecting scissors | More precise than blunt-tipped scissors. Should be kept in a secure location. | ||
| TG-7 or similar camera | Olympus | Camera with macro setting to take voucher photos |
References
- Potts, S. G., et al. Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol. 25 (6), 345-353 (2010).
- IPBES. The Assessment Report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services on Pollinators. , Pollination and Food Production. Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. Bonn, Germany. (2016).
- Goulson, D., Nicholls, E., Botias, C., Rotheray, E. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science. 347 (6229), 1255957(2015).
- Zattara, E. E., Aizen, M. A. Worldwide occurrence records suggest a global decline in bee species richness. One Earth. 4 (1), 114-123 (2021).
- Allen-Wardell, A. G., et al. The potential consequences of pollinator declines on the conservation of biodiversity and stability of food crop yields. Conserv Biol. 12 (1), 8-17 (1998).
- Nieto, A., et al. European Red List of Bees. , Publication Office of the European Union. Luxembourg. (2014).
- Simpson, D. T., et al. Many bee species, including rare species, are important for function of entire plant-pollinator networks. Proc R Soc B. 289 (1972), 20212689(2022).
- Roulston, T. H., Smith, S. A., Brewster, A. L. A comparison of pan trap and intensive net sampling techniques for documenting a bee (Hymenoptera: Apiformes) fauna. J Kans Entomol Soc. 80 (2), 179-181 (2007).
- Gibbs, J., et al. Does passive sampling accurately reflect the bee (Apoidea: Anthophila) communities pollinating apple and sour cherry orchards. Environ Entomol. 46 (3), 579-588 (2017).
- Portman, Z. M., Bruninga-Socolar, B., Cariveau, D. P. The state of bee monitoring in the United States: a call to refocus away from bowl traps and towards more effective methods. Ann Entomol Soc Am. 113 (5), 337-342 (2020).
- Popic, T. J., Davila, Y. C., Wardle, G. M. Evaluation of common methods for sampling invertebrate pollinator assemblages: net sampling out-perform pan traps. PLoS One. 8 (6), e66665(2013).
- Bubnič, J., Mole, K., Prešern, J., Moškrič, A. Non-destructive genotyping of honeybee queens to support selection and breeding. Insects. 11 (12), 896(2020).
- Châline, N., Ratnieks, F. L., Raine, N. E., Badcock, N. S., Burke, T. Non-lethal sampling of honey bee, Apis mellifera, DNA using wing tips. Apidologie. 35, 311-318 (2004).
- Oi, C. A., López-Uribe, M. M., Cervini, M., Del Lama, M. A. Non-lethal method of DNA sampling in euglossine bees supported by mark-recapture experiments and microsatellite genotyping. J Insect Conserv. 17, 1071-1079 (2013).
- Holehouse, K. A., Hammond, R. L., Bourke, A. F. G. Non-lethal sampling of DNA from bumble bees for conservation genetics. Insectes Soc. 50, 277-285 (2003).
- Boyle, N. K., et al. A nonlethal method to examine non-Apis bees for mark-capture research. J Insect Sci. 18, 10(2018).
- Curran, M. F., et al. Use of 3-dimensional videography as a non-lethal way to improve visual insect sampling. Land. 9 (10), 340(2020).
- Austin, G. H. Effect of carbon dioxide anaesthesia on bee behaviour and expectation of life. Bee World. 36 (3), 45-47 (1955).
- Switzer, C. M., Combes, S. A. Bombus impatiens (Hymenoptera: Apidae) display reduced pollen foraging behavior when marked with bee tags vs. paint. J Melittology. 62, 1-13 (2016).
- Ribbands, C. R. Changes in the behaviour of honey bees following their recovery from anaesthesia. J Exp Biol. 27 (3-4), 302-310 (1950).
- Poissonnier, L. A., Jackson, A. L., Tanner, C. J. Cold and CO2 narcosis have long-lasting and dissimilar effects on Bombus terrestris. Insectes Soc. 62, 291-298 (2015).
- Wilson, E. E., Holway, D., Nieh, J. C. Cold anaesthesia decreases foraging recruitment in the New World bumblebee, Bombus occidentalis. J Apic Res. 45 (4), 169-172 (2006).
- Chauzat, M. P., Faucon, J. P. Pesticide residues in beeswax samples collected from honey bee colonies (Apis mellifera l) in France. Pest Manage Sci. 63 (11), 1100-1106 (2007).
- Jha, S., Stefanovich, L., Kremen, C. Bumble bee pollen use and preference across spatial scales in human-altered landscapes. Ecol Entomol. 38 (6), 570-579 (2013).
- Popic, T. J., Wardle, G. M., Davila, Y. C. Flower-visitor networks only partially predict the function of pollen transport by bees. Austral Ecol. 38 (1), 76-86 (2013).
- Bell, K. L., et al. Applying pollen DNA metabarcoding to the study of plant-pollinator interactions. Appl Plant Sci. 5 (6), 1600124(2017).
- Wood, T. J., Kaplan, I., Szendrei, Z. Wild bee pollen diets reveal patterns of seasonal foraging resources for honey bees. Front Ecol Evol. 6, 210(2018).
- Friedle, C., Wallner, K., Rosenkranz, P., Martens, D., Vetter, W. Pesticide residues in daily bee pollen samples (April-July) from an intensive agricultural region in Southern Germany. Environ Sci Pollut R. 28, 22789-22803 (2021).
- Lau, P., Lesne, P., Grebenok, R. J., Rangel, J., Behmer, S. T. Assessing pollen nutrient content: a unifying approach for the study of bee nutritional ecology. Phil Trans R Soc B. 377, 20210510(2022).
- Potter, C., et al. Pollen metabarcoding reveals broad and species-specific resource use by urban bees. PeerJ. 7, e5999(2019).
- Graham, J., Campbell, J., Tsalickis, A., Stanley-Stahr, C., Ellis, J. Observing bees and wasps: Why surveys and monitoring programs are critical and how they can improve our understanding of these beneficial hymenopterans. J Pollinat Ecol. 33, 139-169 (2023).
- Rightmyer, M. G., Deyrup, M., Ascher, J. S., Griswold, T. Osmia species (Hymenoptera, Megachilidae) from the southeastern United States with modified facial hairs: taxonomy, host plants, and conservation status. ZooKeys. 148, 257-278 (2011).
- Michener, C. D., Deyrup, M. Caupolicana from Florida (Hymenoptera: Colletidae). J Kansas Entomol Soc. 77 (4), 774-782 (2004).
- Michener, C. D. Bees of the World. , The Johns Hopkins University Press. Baltimore. (2007).
- Thorp, R. W. The collection of pollen by bees. Pl Syst Evol. 222, 211-223 (2000).
- Streinzer, M., Kelber, C., Pfabigan, S., Kleineidam, C. J., Spaethe, J. Sexual dimorphism in the olfactory system of a solitary and a eusocial bee species. J Comp Neurol. 521 (12), 2742-2755 (2013).
- Marshall, S. A. Field photography and the democratization of arthropod taxonomy. Am Entomol. 54 (4), 207-210 (2008).
- Eberhard, W. G. Sexual SelectionandAnimal Genitalia. , Harvard University Press. Cambridge, MA. (1985).
- Yassin, A. Unresolved questions in genitalia coevolution: bridging taxonomy, speciation, and developmental genetics. Org Divers Evol. 16, 681-688 (2016).
- Magoga, G., et al. Curation of a reference database of COI sequences for insect identification through DNA metabarcoding: COins. Database. 2022, baac055(2022).
- Badirli, S., et al. Classifying the unknown: Insect identification with deep hierarchical Bayesian learning. Methods Ecol Evol. 14 (6), 1515-1530 (2023).
- Guedot, C., Bosch, J., Kemp, W. P. Relationship between body size and homing ability in the genus Osmia (Hymenoptera: Megachilidae). Ecol Entomol. 34 (1), 158-161 (2009).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved