Experimental Infection of Mice with the Parasitic Nematode Strongyloides ratti
In This Article
Summary
Strongyloides ratti is a parasitic nematode that causes transient infections in laboratory mice, displaying tissue-migrating and intestinal life stages. Here, we present a protocol for the maintenance of the parasite cycle in rats and experimental infection of mice, including parasite quantification in the head, lung, and intestine.
Abstract
Strongyloides ratti is a parasitic nematode that naturally infects wild rats. However, most laboratory rat and mouse strains are fully susceptible to infection. Immunocompetent BALB/c and C57BL/6 mice terminate S. ratti infections within a month in the context of a canonical type 2 immune response and remain semi-resistant to a re-infection. The course of infection can be divided into three phases: (a) the tissue migration phase of the infective third-stage larvae during the first two days; (b) the early intestinal phase, including the molting to the adult parasites and embedding in the mucosa of the intestine in days 3 to 6 post-infection with reproduction starting by day 5 to 6 post-infection; (c) the later intestinal phase ending with the complete clearance of the parasites. Experimental infections of mice with S. ratti enable the precise study of host-parasite interactions throughout the whole life cycle at the different sites of infection, as well as immune evasion strategies employed by the parasite. The protocol presented here describes the maintenance of the parasite in Wistar rats, the infection of laboratory mice, and the detection and quantification of S. ratti parasites in the tissue migrating phase and during the intestinal phase.
Introduction
The soil-transmitted helminth Strongyloides stercoralis causes Strongyloidiasis, a disease often referred to as the most neglected among the neglected tropical diseases1. Estimates from 2020 suggest 600 million infections with S. stercoralis worldwide2. Hypothesis-driven laboratory research on S. stercoralis is limited since the helminth is not able to develop past the third-stage larvae (L3) in mice3,4. Hence, the rodent-specific nematode Strongyloides ratti is commonly used for in vivo infection studies in laboratory mice5,6. S. ratti is a natural parasite of wild rats, but most laboratory mouse strains are fully susceptible to infection7. This enables the study of host-pathogen interaction and immune responses throughout different tissues and live stages of the helminth.
The laboratory cycle of S. ratti in mice can be divided into three main phases. After subcutaneous injection of a defined number of infective third-stage larvae (iL3), usually 1000 to 2000, the majority (around 90 %) of the surviving L3 migrate to the head of the mice during the first two days of infection, and only a very small fraction (around 10 %) is retrieved in the lung8. S. ratti actively penetrates the skin of its rodent host. It is possible to mimic this natural infection route in the laboratory by placing a drop of water containing iL3 on the skin of mice and allowing active percutaneous infection9. However, the infection efficacy is much lower, and control of the exact infection dose is not possible. The exact migration route taken by S. ratti iL3, either after percutaneous or subcutaneous (s.c.) infection, remains unknown. However, as no S. ratti DNA can be detected in the blood or organs well supplied with blood, like the kidneys of infected mice8, the primary migration route may not include the bloodstream. Still, S. ratti does not migrate randomly throughout the tissues. Rather, several studies using histology in mice3,10 and rats11 or quantification of viable L3 and S. ratti-derived DNA retrieved from various tissues8 provide evidence that S. ratti L3 migrates from the site of infection directly through skin and muscle tissue predominantly to the nasofrontal region of the head.
Roughly 10 % of the injected larvae survive the tissue migration and reach the head in C57BL/6 and BALB/c mice that display comparable L3 numbers in the head tissue12. Larvae are thought to be swallowed to reach the intestine by day 3 post-infection (p.i.). They embed into the mucosa of the small intestine, molt to adult female parasites, and start reproducing by parthenogenesis by day 5 to day 6 p.i.13. Eggs, but mostly already hatched first-stage larvae (L1), are released into the intestine and are secreted with feces. The peak of the number of adult females is reached around day 6 p.i.14. Interestingly, C57BL/6 mice display a 2- to 5-fold higher intestinal parasite burden than BALB/c mice despite comparable numbers of tissue-migrating L3 in the head in both mouse strains12. During the first week, the intestinal parasite burden in immunocompetent C57BL/6 mice and RAG1 knock-out (KO) mice that lack B and T cells is alike, suggesting that early parasite control is mediated through innate immunity12,15. In the last phase, the parasites are expelled from the intestine within 2 to 4 weeks p.i. in immunocompetent mice (reviewed in6). RAG1 KO mice are not able to clear the infection and contain low numbers of viable and reproducing female adults in the small intestine for up to 1 year12.
After a resolved first infection or immunization with irradiated L3, immunocompetent C57BL/6 and BALB/c mice are semi-resistant to re-infection. Only 1 % of the initial inoculated iL3 reach the head, and only approximately 1-5 adult parasites can be retrieved from the intestine during a second infection8. Thus, the use of the laboratory infection of mice with S. ratti provides a tool to study the impact of the innate as well as adaptive immune system during the complete life cycle of an intestinal nematode with tissue migration phases.
In this manuscript, we provide a detailed description of the maintenance of the S. ratti life cycle in Wistar rats as well as experimental infection of mice and quantification of parasite burden at the different sites of infection. By the exact quantification of S. ratti parasite burden in the head and lung tissue as well as in the intestine of experimental mice, it is possible to dissect the role of certain immune effectors against either the tissue-migrating or the intestinal life stage of this parasitic nematode. Immune responses in wildtype mice and mice lacking specific immune effector cells, receptors, or mediators of interest can be compared as explained in detail in the discussion5,6.
Protocol
Animal experiments were conducted in accordance with the German Animal Welfare Act, and experimental protocols were approved by the German authority (Behörde für Gesundheit und Verbraucherschutz) of the State of Hamburg. Figure 1 provides an overview of the maintenance of S. ratti's life cycle in Wistar rats and the production of iL3 for infection of experimental mice or rats.
1. Preparation of infectious larvae
- Prepare the Baermann apparatus as displayed in Figure 1. Close the bottom of the hose with a clamp at an oblique angle and place a tissue wipe in the sieve. Fill with lukewarm water (approximately 35-37 °C) until the sieve is covered in water.
- Fill the sieve with the feces-charcoal mix prepared as described below (step 6). The mix needs to be completely covered by water.
- Switch on the light directly behind the Baermann apparatus. Viable iL3 will migrate actively through the wipe.
- After 30 min the larvae have settled above the clamp, collect them into a 50 mL tube by opening the clamp shortly. Collect the whole pellet, but ensure the volume is as small as possible.
- Fill the 50 mL tube with PBS containing 1% Penicillin-Streptomycin (PBS/Pen-Strep) and let the larvae settle at the bottom of the tube by gravity at 4 °C for 30 min. Carefully remove the supernatant with a pipette and repeat the washing step 3x.
- After the third washing step, resuspend the pellet in 30 to 50 mL PBS/Pen-Strep, depending on the pellet size. Transfer 1 µL drops of the solution to a microscopy slide. Agitate the solution immediately before pipetting since the iL3 settles down quickly.
- Inspect the droplets under an inverse microscope at 40x magnification. The iL3 should be moving vividly. Count the iL3 per 1 µL droplet in 5 to 10 replicates and calculate the mean per µL.
2. Infection of mice
NOTE: Prepared iL3 can be stored in PBS/Pen-Strep at 4 °C in a 50 mL tube that should be kept horizontally to avoid damaging the iL3. Viability in vitro indicated by the vivid movement of iL3 is unchanged for up to 1 week in our hands. As there is no systematic comparison of the infectivity of L3 after different storage times, we use freshly prepared iL3 and stored iL3 for a maximum of 24 h after preparation as an internal standard. Infections with older iL3 batches are possible. However, it is mandatory to use the same iL3 batch for infection of different groups within one experiment. Infections of mice should preferably be done by two persons, with one person holding the mouse and another performing the injection.
- Prepare one 1.5 mL centrifugation tube per mouse containing a total of 1000 iL3 (C57BL/6) or 2000 iL3 (BALB/c) counted as described above in step 1.7 in PBS/Pen-Strep that were prepared using the Baermann apparatus as explained above (step 1). Agitate thoroughly between the pipetting steps as the iL3 settles quickly.
- Let the iL3 settle by gravity for 20-30 min at room temperature (RT) and aspirate the supernatant with a 0.5 mL syringe as completely as possible, leaving around 30 µL of iL3 suspension in the tube. Resuspend the iL3 with a 0.5 mL syringe (28G) or by flicking the tube and aspirate the remaining suspension.
NOTE: The iL3 are less likely to stick to wet plastic than the dry syringe. Hence, it is advantageous to use the same syringe to take off the supernatant. - Mice do not need to be anesthetized for infection. Pick up the mice with the scruff and grab one hindfoot of the mice. Use female and male C57BL/6 of more than 8 weeks of age and at least 20 g weight. Inject the entire solution containing the iL3 subcutaneously into the footpad at a flat angle. Slowly retract the syringe to avoid spilling larvae suspension from the puncture site. A very small dome containing the liquid will form. This will be absorbed by itself.
NOTE: Subcutaneous infection into the footpad rather than the flank or nape of the neck mimics the natural infection by iL3, which lives in moist earth fertilized with feces and actively penetrates the intact skin. Also, the first draining lymph node (LN) is defined as the popliteal LN. This LN can be used for the analysis of early (i.e., day 2 p.i.) immunological changes, for instance, expansion of Foxp3+ regulatory T cells in response to infection, as shown in16.
3. Counting of iL3 in the head and lung of infected mice
- For analysis of larvae burden in the tissue, sacrifice the mice on day 1-3 p.i. as the peak of the larvae burden is at day 2 p.i.8,12. Perform euthanasia by CO2 overdose narcosis. After the absence of cornea and interdigital reflexes, perform cervical dislocation.
- Spray the abdomen and neck with a commercial disinfectant and cut the skin over the abdomen with scissors. Pull the skin back to reveal the anterior abdominal wall and cut along the midline to open the peritoneal cavity. Cut the diaphragm and cut open the ribs to both sides to expose the lung in the pleural cavity.
- Collect the lung lobes in a 24-well plate divided by lines containing 1 mL of tap water. Draw or print the lines on the plate at a distance of around 4 mm. The distance between the lines should make it possible to see both lines at the same time at 40x magnification.
- Cut the entire lung into six pieces of approximately 0.75 cm x 0.75 cm.
- Cut off the head with bone scissors. Remove the skin and fur using the fingers. Try to remove as little muscle tissue as possible.
- Dissect the complete head (including brain and bones) into four quarters behind the eyes and longitudinally through the middle of the head (see Figure 2C). Place the anterior and posterior quadrants in a well of a 6-well plate marked by lines containing 2 mL of tap water. Place the tissue with the cuts facing down into the water.
NOTE: Usually, this approach gives an exact recording of the numbers of head tissue-migrating larvae. If a separate analysis of L3 numbers in the brain is needed step 3.7 may be included. - If a separate analysis of the brain is desired, remove the skin from the head using the fingers. Then, use scissors to drill through the skullcap between the eyes. From this incision, open the top of the skull sagittal with unused or thoroughly cleaned scissors. To expose the brain, open the right and left skullcaps with a tweezer. Use a spatula to lift the brain from the anterior side upwards and the nerve cords are cut with clean scissors. Remove the brain and transfer it to a 24-well plate marked by lines containing tap water.
- Place the plates in an incubator at 37 °C. Incubate for 3 h while swirling the plates every hour (h) 10x. After the incubation, swirl one last time, remove the remaining tissue parts with forceps from the wells, and discard the tissue.
- Completely count every larvae in the remaining water in the wells under an inverted microscope with a 40x magnification by moving along the lines drawn on the well bottoms. Count the tissue-migrating larvae on the same day to increase the liability of the results.
4. Counting of S. ratti parasites in the intestine of infected mice
- For analysis of the parasite burden in the intestine sacrifice the mice on the day p.i. of interest, use day 3-21 p.i. for a complete kinetic. The peak of the parasite burden in C57BL/6 and BALB/c mice within the small intestine is at day 6 p.i.14. Perform euthanasia by CO2 overdose narcosis. After the absence of cornea and interdigital reflexes, perform a cervical dislocation.
- Soak the abdominal region of the mice in disinfectant and cut the skin over the abdomen. Pull the skin back to reveal the anterior abdominal wall and cut along the midline to open the peritoneal cavity.
- Remove the entire intestine by cutting between the stomach and the proximal duodenum, as well as between the colon and the anus (see Figure 3B). Gently pull out the intestine with fingers and place it in a Petri dish containing tap water.
- Cut the intestine open longitudinally and wash out the feces and mucus by shaking vigorously in tap water for at least 10 s. A minor fraction of adult parasites and some L1 will get washed out, but viable L4 and adult S. ratti parasites are embedded in the mucosa of the intestine in wild-type (WT) mice and will not be removed by this washing step.
- If of interest, divide the intestine further as described below.
- Isolate the duodenum by an incision after the second Peyer's patch (approximately 2-3 cm). Isolate the ileum by two incisions, one at the first and the other at the second Peyer's patch (approximately 1-2 cm). Isolate the jejunum after isolation of the duodenum and ileum as this is the remaining section.
- Divide the jejunum into three equal sections to allow a very exact definition of parasite localization. Isolate the caecum between the ileum and the colon. Isolate the colon by cutting after the caecum. The majority of adult parasites can be found in the first third of the small intestine (see Figure 3B).
- Transfer the cleaned intestinal parts into 50 mL tubes containing 20 mL of tap water. Place horizontally in the 37 °C incubator for 3 h. Shake very vigorously for 10 s every hour.
- After 3 h of incubation, remove the tissue from the water. Place the tubes vertically at RT, and the parasites sediment by gravity in 30 min.
- Remove the supernatant until around 5 mL of water is left. Fill up to 25 mL with tap water to repeat the washing step. For analysis of the small intestine, repeat this washing step another time to have good visibility under the microscope.
- When good visibility is achieved, aspirate the supernatant until around 5 mL of water is left and transfer the liquid into two wells per mouse intestine in a 6-well plate marked by lines, as explained for counting larvae in the head.
- Count the adult female parasites under an inverted microscope with a 40x magnification by moving along the lines drawn on the well bottoms. Count parasites on the day of sacrification. Count the parasites as quickly as possible, as prolonged incubation in water will lead to their death and disintegration.
NOTE: In addition to fourth-stage larvae and adult parasites, L1 can also be detected here from around day 5 p.i. These are much smaller and much more abundant than L4 and adult parasites and should be distinguished easily.
5. S. ratti maintenance in Wistar rats
NOTE: Rat infections should preferably be done by two people, with one person holding the rats and another performing the injection. To maintain the parasite cycle, 4-8-weeks-old Wistar rats are infected.
- Prepare one 1.5 mL centrifugation tube per rat containing 2500 iL3 in PBS/Pen-Strep using the Baermann apparatus as explained before. Agitate thoroughly between the pipetting steps as the larvae settle quickly.
- Let the larvae settle by gravity for 20-30 min (RT) and aspirate the supernatant in a 0.5 mL syringe (28G) until around 200 µL of larvae suspension is left.
- For infections, place the rats in the arm crook of one person and fix the head gently and carefully there. The other person gently grasps the flank of the rat and injects the resuspended iL3 suspension subcutaneously into the nuchal fold.
6. Charcoal culture
- Hold infected rats in cages containing several layers of cellulose and less litter starting at days 5 and 12 after the infection. This exchange of litter for several layers of cellulose is done in order to make feces collection easier compared to collection of feces out of litter
- At days 6-8 and 13-15 p.i., transfer the rats into a fresh cage and collect all the feces from the old cage by picking up the feces pellets from the cages after transferring the rats to fresh cages and pooling them from one cage into a 50 mL tube. Collect feces for 2 weeks p.i. before the establishment of the immune response reduces the larval burden in the feces. After 2 weeks p.i. the rats are euthanized via CO2 overdose narcosis. After the absence of cornea and interdigital reflexes, perform a cervical dislocation.
NOTE: Immunocompromised rats and Mongolian gerbils maintain S. ratti infections for longer periods17,18. Studying immune response and immunomodulation during S. ratti infection, we decided to maintain the cycle in immunocompetent hosts to maintain the selection pressure of intact host immunity. - Use the collected feces samples to prepare charcoal cultures, which will allow the development of iL3 from the L1 present in the feces.
- Presoak the charcoal with tap water. Before using charcoal for cultures, fill it into a sieve and wash it under running tap water until the water runs clear. Keep the charcoal moist at all times. Soak the charcoal for at least 24 h before use.
- Mix the feces with presoaked activated charcoal to a ratio of approximately 1:1. Arrange the mixture with a gradient diagonally and cover it with another layer of activated charcoal in a flat glass beaker to reach a final ratio of 1:2.
NOTE: Incubation of the feces with presoaked activated charcoal mimics the moist soil in which iL3 develops in nature while preventing excessive fungal contamination. - Cover the glass vessel with a transparent film. Make sure there are some air holes to allow air circulation.
- Incubate for 6-7 days at 25 °C at around 90 % humidity without CO2. Make sure the incubator contains a water vessel to keep the cultures moist. Keep the cultures for up to 14 days at 25 °C if needed. iL3 will develop either directly or indirectly19 and stay arrested in the iL3 stage until infection.
Results
S. ratti migrates from the site of infection predominantly to the head and later to the intestine on a route that is not exactly defined. To investigate the exact localization in the head tissue and the intestine, C57BL/6 mice were infected with 1000 iL3 into the left hind foot pad. Mice were sacrificed on day 1 to day 14 p.i. and S. ratti parasites were quantified in the anterior and posterior head, brain, and lung (Figure 2) as well as in the duodenum, jejunum, ileum, caecum, and colon (Figure 3).
At day 1 p.i., the first L3 reached the head, showing a uniform distribution (Figure 2A). In addition, a small fraction of larvae was retrieved in the lungs on day 1 p.i. (Figure 2B). L3 numbers increased markedly on day 2 p.i. to an average of 174 L3 ± 13 in the head and an average of 17 ± 1.7 in the lung. Thus, the majority of L3 (around 90%) is retrieved from the head and only around 10% from the lungs. Most L3 (mean 93 ± 13.3) were localized in the anterior side of the head, but also around 41 ± 4.7 L3 larvae were localized in the posterior side of the head and around 39 ± 5.8 in the brain. In line with this observation, the presence of L3 in the brain and the cerebrospinal fluid was reported at 24 h p.i. and with a maximum at 48 h p.i. in C57BL/6 mice after percutaneous infection20. A distinct decrease in the total L3 numbers was observed on day 3 p.i. compared to the larvae burden on day 2 p.i. and no L3 were retrieved from head tissue on day 4 p.i. (Figure 2A). Accordingly, the arrival of S. ratti parasites was detected in the intestine on day 4 p.i. (Figure 3A). To allow a precise definition of parasite localization, the intestine was subdivided into distinct segments, i.e., duodenum, jejunum, ileum, caecum, and colon (Figure 3B). On day 4, p.i., the majority of S. ratti parasites were localized in the duodenum and the first two-thirds of the jejunum (Figure 3A). This localization was consistent until day 6 p.i. From day 7 p.i. onward, the majority of S. ratti adults were localized in the caecum, where they persisted until day 9 p.i. (Figure 3A). Parasite numbers in the caecum dropped by day 11 p.i. to around 20 and to 0 by day 14 p.i. We did not retrieve significant numbers of parasites from the remaining colon at any time point analyzed except approximately 5-11 parasites by day 11 p.i. While the localization of S. ratti parasites changed from day 4 to day 9 p.i. and the majority of adults were ejected from the small intestine after day 7 p.i., the total numbers retrieved from the whole intestine remained constant until day 9. Viable L1 was not quantified but is detectable from day 4 to day 11, with peak on day 6 (data not shown).
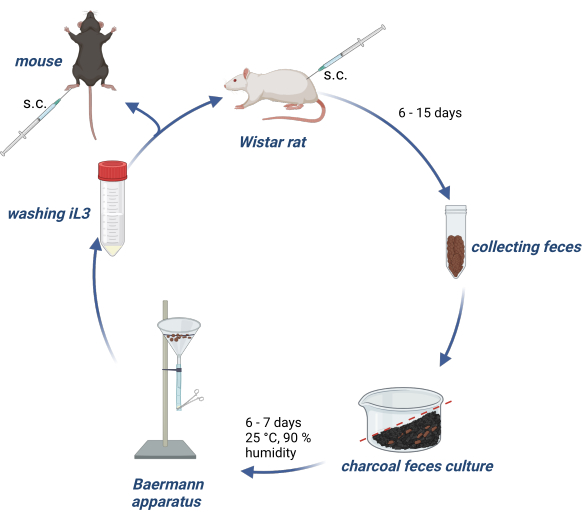
Figure 1: Maintenance of S. ratti life cycle in rats and mice. Wistar rats are injected s.c. into the nuchal fold with 2500 iL3. After 6- 15 days, p.i. their feces are collected, mixed with water-soaked activated charcoal, arranged with a gradient and covered with a transparent film, including air holes. This culture is incubated for 6-7 days at 25 °C and 90% humidity. The iL3 are isolated using the Baermann apparatus and washed 3x with PBS/Pen-Strep. Experimental mice are injected with 1000 iL3 subcutaneously into the hind footpad. Created with BioRender.com. Figure 1 was created in BioRender. Linnemann, L. (2024) https://BioRender.com/g80l370. Please click here to view a larger version of this figure.
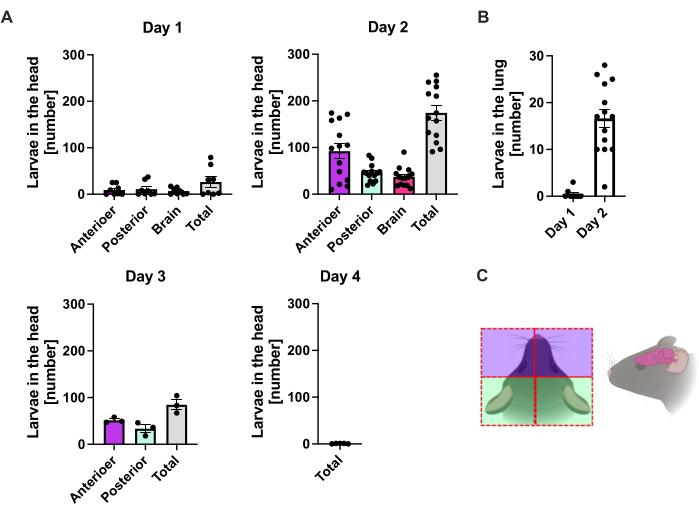
Figure 2: Quantification of S. ratti in the lung and head tissue over time. C57BL/6 mice were s.c. injected with 1000 iL3 in the hind foot pad. Mice were sacrificed at the indicated time points, and S. ratti parasites in (A) head and (B) lung were counted. Each symbol represents the L3 counts of an individual mouse; the bar graph shows the mean value, and error bars indicate SEM. The graphs show combined data from individual experiments. Day 1: two independent experiments with n=4 per time point and experiment; day 2: four independent experiments with n=4, n=4, n=6, and n=3; day 3: two independent experiments with n=4 and n=3; day 4: one experiment with n=5. (C) Schematic cartoon showing the individual regions of the head used for isolation. The red dashed line indicates the incision path. Figure 2C was created in BioRender. Linnemann, L. (2024) https://BioRender.com/t83e660. Please click here to view a larger version of this figure.
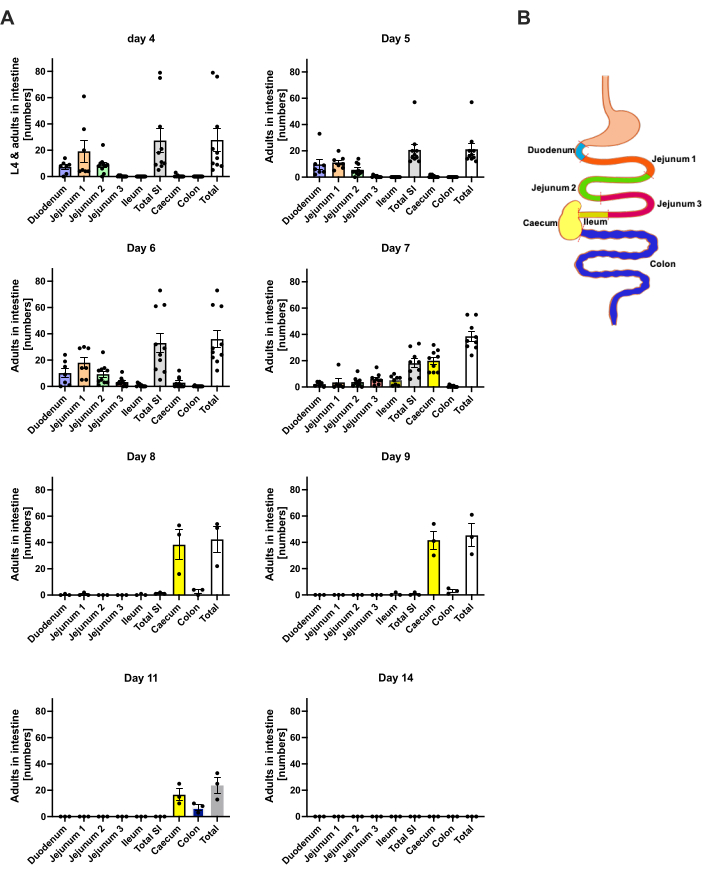
Figure 3: Quantification of S. ratti in the intestine regions over time. Male C57BL/6 mice were s.c. injected with 1000 iL3 in the hind foot pad. (A) Mice were sacrificed at the indicated time points, and S. ratti parasites except L1 were counted in the following regions: duodenum, jejunum 1-3, ileum, caecum, and colon. (B) Schematic overview of the different intestinal regions. Each symbol represents the parasite counts of an individual mouse; the bar graph shows the mean, and error bars indicate SEM. Shown is combined data from individual experiments. Day 4: two independent experiments with n=4 and n=6 mice; Day 5: two independent experiments with n=4 and n=6; Day 6: two independent experiments with n=4 and n=6; Day 7: two independent experiments with n=6 and n=3; Days 8 to 14: one experiment with n=3. SI: small intestine. Figure 3B was created in BioRender. Linnemann, L. (2024) https://BioRender.com/h27y297. Please click here to view a larger version of this figure.
Discussion
The infection of experimental mice with S. ratti represents an excellent model to study the nature of protective immune responses to helminth infections at multiple sites and stages of immunity. Using different KO mouse lines and cell- or cytokine-depletion models, the role of specific immune cells, mediators, or receptors can be studied in an acute helminth infection model. The possibility to quantify the parasite burden in the head as well as in the intestine offers the possibility to differentiate the role of immune cells and effectors in various tissues and phases during the parasite's life cycle. Depletion of specific cell types through antibody injections allows the study of their role, specifically during the intestinal immune response if the depleting treatment starts after the tissue migrating phase is completed. If desired, the intestine can further be subdivided into duodenum, jejunum, ileum, caecum, and colon to detect even minor shifts in the parasite localization or clearance kinetics. It should be noted that the inter- and intra-experimental variation in these infection experiments, even in inbred mice, is rather high, reflecting the variation introduced by the interaction of parasite and host as well as different batches of S. ratti L3 displaying different infection efficacy (see Figure 2 and Figure 3). To reduce variability, the age and sex of the experimental mice should be similar. Moreover, if KO and WT mice are compared, it is highly advisable to use littermate controls instead of WT mice derived from an independent breeding colony. Nevertheless, if sufficient sample sizes are used, it is possible to generate reliable results comparing parasite burden in mice deficient or competent for certain effectors, leading to a clear picture of immune effectors involved in the protective immune response to S. ratti (reviewed in 6).
A key distinguishing feature of S. ratti compared to other nematode infection models, such as the closely related S. venezuelensis or N. brasiliensis, is the larvae's unique route of migration within the host. Unlike N. brasiliensis and S. venezuelensis, whose life cycles both contain a pulmonary phase21,22,23 S. ratti primarily bypasses the lung and migrates through the muscle and skin tissue to the head8,12. Only approximately 10% of the surviving parasites on day 2 p.i. are found in the lungs. Meanwhile, the location of S. ratti in the head focuses on the nasofrontal region, in line with previous studies8. These characteristics make S. ratti a valuable model for studying the host-parasite interactions, specifically in skin and muscle tissue as well as in the tissue draining lymph nodes and enables studies on the immune responses that may be obscured or complicated by an extended pulmonary involvement as in other nematode infection models. Strikingly, S. ratti L3 is also retrieved from the cerebrospinal fluid20 and the brain (Figure 2A), although infection-induced neurological symptoms or death are relatively rare and have never been observed in our animal facility. Future research may elucidate if these brain-localized parasites are trapped or if a path to the intestine exists.
The genus Strongyloides also has the unique ability to form free-living generations between the parasitic generations24. These free-living stage of S. ratti, as well as its reproduction by pathogenesis, furthermore facilitates the generation of transgenic larvae. The use of microinjections into free-living females enabled the generation of larvae expressing model antigens like 2W1S fused to a green fluorescent protein. While the expression of the epitope was lost during molting to adults, it enabled the tracking and characterization of S. ratti-specific CD4+ T cells in the lung and lung-draining mediastinal lymph nodes25. This approach provides an excellent tool for studying CD4+ T cell biology in the context of helminth infections and anti-helminth vaccine development.
S. ratti is a versatile model organism for immunological research of helminth parasites that display tissue migrating and intestinal life stages in general. Human S. stercoralis infections are marked by extreme chronicity due to the occurring autoinfection, which may also lead to hyperinfection syndrome in immunosuppressed hosts, mostly patients receiving glucocorticoid therapy post-transplantation26. It should be noted that this aspect of autoinfection and hyperinfection is difficult to model in mice. Neither S. ratti-infected RAG1 KO nor nude mice5,27 are susceptible to hyperinfection. Of note, one murine model of hyperinfection with S. stercoralis was established using glucocorticosteroid-treated severely immunocompromised mice (NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ), which may allow the analysis of at least aspects of the hyperinfection syndrome in mice eventually28.
Nevertheless, studies utilizing S. ratti infection in mice demonstrated that eosinophils and neutrophils play a non-redundant role in eradicating tissue-migrating larvae. The depletion or absence in genetically modified mice resulted in elevated L3 numbers in the head8. While mast cells and basophilic granulocytes were dispensable during the tissue migration phase, both mast cells and basophils contributed to controlling intestinal parasite burden. Their absence did not affect L3 numbers in the tissue but elevated the numbers of adult S. ratti parasites in the intestine on day 6 p.i.12,29. Further analysis revealed that the absence of basophils or selectively connective-tissue mast cells allowed infection termination with WT kinetics. By contrast, mice lacking connective tissue and mucosal mast cells remained infected for 20 weeks12. These findings unveiled a pivotal role for mucosal mast cells in final infection termination, underscoring the value of this infection model in elucidating the function of mucosal mast cells during helminth infection. The further definition of potential changes in the intestinal localization of S. ratti parasites in the absence of certain immunological effector cells will help to define their function in anti-helminth immunity even more precisely.
Furthermore, immune-evasive mechanisms employed by helminths to facilitate their survival may be studied in this system. It was shown that depletion of Foxp3+ regulatory T cells or deletion of a regulatory receptor on effector T cells, which were both induced during S. ratti infection, reduced the parasite burden day 6 p.i. and larval output throughout infection15,16,30,31. Furthermore, it was possible to define the intestine as the tissue targeted by immune evasion and IL-9-mediated mast cell activation as the immune pathway suppressed. Finally, the mechanism of ILC2-mediated initiation of type 2 immunity by tissue-derived alarmin cytokines such as IL-33 can be studied using suppressors and enhancers of endogenous IL-3332.
The isolation of large numbers of iL3 via the Baermann presents the possibility for further in vitro studies. Co-cultures of L3 with immune cells or potential drug candidates enable direct investigation of the effects on L3 viability and motility. Ex vivo restimulation of cells isolated from infected mice with S. ratti antigen lysate or viable L3 provides a platform to study cytokine production across various cell types. Finally, protein and lipid fractions of L3 may be used for the identification of S. ratti-derived pathogen-associated molecular patterns or immunomodulatory effector molecules
As helminth infections still present a major health burden globally, research to further elucidate the immune responses induced by helminths and the evasion mechanism employed by the parasites remains pivotal to improving treatment options and developing preventive strategies such as vaccinations. S. ratti infection in mice presents a versatile model for research on helminth-host interactions during an acute infection model.
Disclosures
The authors have no conflict of interest. The language model Perplexity AI 2024 was used to revise text drafts and improve formulations.
Acknowledgements
This work was supported by the Jürgen Manchot Foundation and the German Research Association (Grant BRE 3754/6-1 and BRE 3754/10-1). Figures 1, 2C, and 3B were created in BioRender.com.
Materials
| Name | Company | Catalog Number | Comments |
| 50 ml tubes | Sarstedt, N mbrecht, DE mbrecht, DE | 6,25,47,254 | 50 ml https://www.sarstedt.com/produkte/labor/reagenz-zentrifugenroehren/roehren/produkt/62.547.254/ |
| BD Micro-Fine U100 Insulin 0.5 ml | BD Bioscience | 7468077 | 0.5 ml https://www.bestimed.de/bd-micro-fine-insulinspritze-05-ml-u100-8-mm-100x05ml-324825.html |
| centrifugeation tubes | Sarstedt, Nümbrecht, DE | 72,706 | 1.5 ml https://www.sarstedt.com/produkte/labor/mikro-schraubroehren-reagiergefaesse/reagiergefaesse/produkt/72.706/ |
| Charcoal | Roth | 0998.3 | 5 kg https://www.carlroth.com/de/de/aktivkohle/aktivkohle/p/0998.3 |
| Falcon 6-well Clear Flat Bottom, not treated cell multiwell culture plate, with Lid, sterile | Corning | 351146 | 6-well https://www.corning.com/emea/de/search.html?_cookie=false &searchText=351146&search-initialcatalog =Corporate+Communications& initialResultType=products |
| Freezer & Refrigerator | Liebherr-Hausgeräte, Rostock, DE | ||
| Greiner Bio-One 24-Well-Platten für Zellkulturen aus Polystyrol | Fisher Scientific | 10177380 | 24-well https://www.fishersci.de/shop/products/polystyrene-24-well-cell-culture-multiwell-plate/10177380#?keyword=24-well |
| Incidin Premium Wipes | Ecolab Healthcare | 100 10 279 | https://www.ecolabhealthcare.de/website/seiten/produkte/flaechendesinfektion/tuecher/incidin_premium_wipes.php |
| Incubator 25°C | Heraeus Instruments, Hanau, DE | ||
| Incubator 37°C | Heraeus Instruments, Hanau, DE | ||
| Microscope | Helmut Hund, Wetzlar, DE | 4 x objectiv lens, 10 x ocular lens | |
| Parafilm M | Parafilm | 11772644 | 4 in. X 125 ft. https://www.fishersci.de/shop/products/parafilm-m-laboratory-wrapping-film-2/11772644 |
| Penicillin/Streptomycin (Pen-Strep) | Capricorn | PS-B | 100x https://www.capricorn-scientific.com/en/shop/penicillin-streptomycin-pen-strep-100x~p1205 |
| ROTI Fair 10x PBS 7.4 | Roth | 1105.1 | https://www.carlroth.com/de/de/fertigloesungen-tabletten-portionsbeutel/rotifair-10x-pbs-7-4/p/1105.1 |
References
- Olsen, A., et al. Strongyloidiasis - the most neglected of the neglected tropical diseases. Trans Royal Soc Tropical Med Hygiene. 103 (10), 967-972 (2009).
- Buonfrate, D., et al. The global prevalence of Strongyloides stercoralis infection. Pathogens. 9 (6), 468 (2020).
- Dawkins, H. J. S., Grove, D. I. Attempts to establish infections with Strongyloides stercoralis in mice and other laboratory animals. J Helminthol. 56 (1), 23-26 (1982).
- Abraham, D., et al. Strongyloides stercoralis: protective immunity to third-stage larvae inBALB/cByJ mice. Exp Parasitol. 80 (2), 297-307 (1995).
- Breloer, M., Abraham, D. Strongyloides infection in rodents: immune response and immune regulation. Parasitology. 144 (3), 295-315 (2017).
- Breloer, M., Linnemann, L. Strongyloides ratti infection in mice: immune response and immune modulation. Philosophical Trans Royal Society B. 379 (1894), 20220440 (2024).
- Dawkins, H. J. S., Grove, D. I., Dunsmore, J. D., Mitchell, G. F. Strongyloides ratti: Susceptibility to infection and resistance to reinfection in inbred strains of mice as assessed by excretion of larvae. Int J Parasitol. 10 (2), 125-129 (1980).
- Ehrens, A., et al. Eosinophils and neutrophils eliminate migrating Strongyloides ratti larvae at the site of infection in the context of extracellular DNA trap formation. Front Immunol. 12, 715766 (2021).
- Eschbach, M. L., et al. Strongyloides ratti infection induces transient nematode-specific Th2 response and reciprocal suppression of IFN-γ production in mice. Parasite Immunol. 32 (5), 370-383 (2010).
- Dawkins, H., Muir, G., Grove, D. Histopathological appearances in primary and secondary infections with Strongyloides ratti in mice. Int J Parasitol. 11 (1), 97-103 (1981).
- Tada, I., Mimori, T., Nakai, M. Migration route of Strongyloides ratti in albino rats. Jap J Parasit. 28 (4), 219-227 (1979).
- Reitz, M., et al. Mucosal mast cells are indispensable for the timely termination of Strongyloides ratti infection. Mucosal Immunol. 10 (2), 481-492 (2017).
- Viney, M. E., Lok, J. B. The biology of Strongyloides spp. WormBook. , 1-17 (2018).
- Reitz, M., et al. Interleukin-9 promotes early mast cell-mediated expulsion of Strongyloides ratti but is dispensable for generation of protective memory. Sci Rep. 8 (1), 8636 (2018).
- Breloer, M., et al. Cutting edge: The BTLA-HVEM regulatory pathway interferes with protective immunity to intestinal Helminth infection. J Immunol. 194 (4), 1413-1416 (2015).
- Blankenhaus, B., et al. Foxp3+ regulatory T cells delay expulsion of intestinal nematodes by suppression of IL-9-driven mast cell activation in BALB/c but not in C57BL/6 mice. PLoS Pathogens. 10 (2), e1003913 (2014).
- Niamatali, S., Nolan, T. J., Schad, G. A. . Can Autoinfection be Provoked in the Strongyloides ratt/-infected Gerbil, Meriones unguiculatus. 59 (2), 149-152 (1992).
- Gardner, M. P., Gems, D., Viney, M. E. Extraordinary plasticity in aging in Strongyloides ratti implies a gene-regulatory mechanism of lifespan evolution. Aging Cell. 5 (4), 315-323 (2006).
- Viney, M. E. Developmental switching in the parasitic nematode Strongyloides ratti. Proc Biol Sci. 263 (1367), 201-208 (1996).
- Dawkins, H. J., Thomason, H. J., Grove, D. I. The occurrence of Strongyloides ratti in the tissues of mice after percutaneous infection. J Helminthol. 56 (1), 45-50 (1982).
- Takamure, A. Migration route of Strongyloides venezuelensis in rodents. Int J Parasitol. 25 (8), 907-911 (1995).
- Filbey, K., Bouchery, T., Le Gros, G. The role of ILC 2 in hookworm infection. Parasite Immunol. 40 (2), e12429 (2018).
- Allen, J. E., Sutherland, T. E. Host protective roles of type 2 immunity: Parasite killing and tissue repair, flip sides of the same coin. Semin Immunol. 26 (4), 329-340 (2014).
- Dulovic, A., Puller, V., Streit, A. Optimizing culture conditions for free-living stages of the nematode parasite Strongyloides ratti. Exp Parasitol. 168, 25-30 (2016).
- Douglas, B., et al. Transgenic expression of a T cell epitope in Strongyloides ratti reveals that helminth-specific CD4+ T cells constitute both Th2 and Treg populations. PLOS Pathogens. 17 (7), e1009709 (2021).
- Vadlamudi, R. S., Chi, D. S., Krishnaswamy, G. Intestinal strongyloidiasis and hyperinfection syndrome. Clin Mol Allergy. 4 (1), 8 (2006).
- Viney, M., Kikuchi, T. Strongyloides ratti and S. venezuelensis- rodent models of Strongyloides infection. Parasitology. 144 (3), 285-294 (2017).
- Patton, J. B., et al. Methylprednisolone acetate induces, and Δ7-dafachronic acid suppresses, Strongyloides stercoralis hyperinfection in NSG mice. Proc Natl Acad Sci. 115 (1), 204-209 (2018).
- Reitz, M., Brunn, M. L., Voehringer, D., Breloer, M. Basophils are dispensable for the establishment of protective adaptive immunity against primary and challenge infection with the intestinal helminth parasite Strongyloides ratti. PLOS Neglected Trop Dis. 12 (11), e000699 (2018).
- Blankenhaus, B., et al. Strongyloides ratti infection induces expansion of Foxp3+ regulatory T cells that interfere with immune response and parasite clearance in BALB/c Mice. J Immunol. 186 (7), 4295-4305 (2011).
- Hartmann, W., Blankenhaus, B., Brunn, M. L., Meiners, J., Breloer, M. Elucidating different pattern of immunoregulation in BALB/c and C57BL/6 mice and their F1 progeny. Sci Rep. 11 (1), 1536 (2021).
- Meiners, J., et al. IL-33 facilitates rapid expulsion of the parasitic nematode Strongyloides ratti from the intestine via ILC2- and IL-9-driven mast cell activation. PLOS Pathogens. 16 (12), e1009121 (2020).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved