Column Chromatography
Overview
Source: Laboratory of Dr. Jimmy Franco - Merrimack College
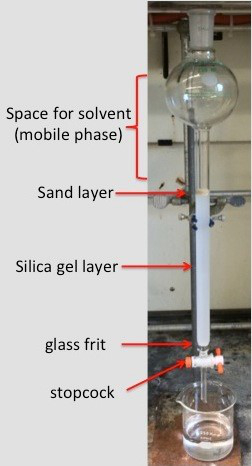
Column chromatography is one of the most useful techniques for purifying compounds. This technique utilizes a stationary phase, which is packed in a column, and a mobile phase that passes through the column. This technique exploits the differences in polarity between compounds, allowing the molecules to be facilely separated.1 The two most common stationary phases for column chromatography are silica gel (SiO2) and alumina (Al2O3), with the most commonly used mobile phases being organic solvents.2 The solvent(s) chosen for the mobile phase are dependent on the polarity of the molecules being purified. Typically more polar compounds require more polar solvents in order to facilitate the passage of the molecules through the stationary phase. Once the purification process has been completed the solvent can be removed from the collected fractions using a rotary evaporator to yield the isolated material.
Procedure
1. Silica Gel Slurry
- Pour the silica gel into an Erlenmeyer flask. The weight of the packing material should be roughly 50x that of the sample being separated. If the compounds being separated have very similar Rf values, then it may require using a larger amount of silica per sample, which is the case in this example.
- Place 10 g of silica in the Erlenmeyer flask, since 50 mg of sample (45 mg of fluorenone and 5 mg of tetraphenylporphyrin) are being isolated.
Results
The sample containing a mixture of tetraphenylporphyrin (TPP, 5 mg) and fluorenone (45 mg) has been successfully separated and each compound has been isolated. The TPP eluted first off the column as a dark purple-reddish band and the fluorenone subsequently eluted off the column as a yellow band (Figure 2). The eluted fractions were collected in test tubes and identified by their distinctive colors (Figure 3). The fractions containing the
Application and Summary
Summary
Column chromatography is a convenient and versatile method for purifying compounds. This method separates compounds based on polarity. By exploiting differences in the polarity of molecules, column chromatography can facilely separate compounds by the rate at which the compounds traverse through the stationary phase of the column. One of the benefits of column chromatography (especially when compared to recrystallization) is that very little about the compounds needs
References
- Mayo, D. W.; Pike, R. M.; Forbes, D. C., Microscale organic laboratory : with multistep and multiscale syntheses. 5th ed.; J. Wiley & Sons: Hoboken, NJ; p xxi, 681 p (2011).
- Armarego, W. L. F.; Chai, C. L. L., Purification of laboratory chemicals. 5th ed.; Butterworth-Heinemann: Amsterdam; Boston; p xv, 609 p (2003).
- Silverman, R. B.; Holladay, M. W., The organic chemistry of drug design and drug action. Third edition / ed.; Elsevier/AP, Academic Press, is an imprint of Elsevier: Amsterdam ; Boston; p xviii, 517 pages (2014).
- Mortensen, D. S.; Perrin-Ninkovic, S. M.; Shevlin, G.; Elsner, J.; Zhao, J.; Whitefield, B. et. al. Optimization of a Series of Triazole Containing Mammalian Target of Rapamycin (mTOR) Kinase Inhibitors and the Discovery of CC-115. Journal of Medicinal Chemistry (2015).
- Davies, D. R.; Johnson, T. M., Isolation of Three Components from Spearmint Oil: An Exercise in Column and Thin-Layer Chromatography. Journal of Chemical Education,84 (2), 318 (2007).
- Taber, D. F.; Hoerrner, R. S., Column chromatography: Isolation of caffeine. Journal of Chemical Education, 68 (1), 73 (1991).
Tags
Skip to...
Videos from this collection:

Now Playing
Column Chromatography
Organic Chemistry
360.6K Views

مقدمة في التحفيز
Organic Chemistry
34.5K Views

Assembly of a Reflux System for Heated Chemical Reactions
Organic Chemistry
168.0K Views

Conducting Reactions Below Room Temperature
Organic Chemistry
70.7K Views

Schlenk Lines Transfer of Solvents
Organic Chemistry
41.7K Views

Degassing Liquids with Freeze-Pump-Thaw Cycling
Organic Chemistry
56.3K Views

Preparing Anhydrous Reagents and Equipment
Organic Chemistry
79.4K Views

Purifying Compounds by Recrystallization
Organic Chemistry
709.6K Views

Separation of Mixtures via Precipitation
Organic Chemistry
157.9K Views

Solid-Liquid Extraction
Organic Chemistry
238.0K Views

Rotary Evaporation to Remove Solvent
Organic Chemistry
212.9K Views

Fractional Distillation
Organic Chemistry
334.7K Views

Growing Crystals for X-ray Diffraction Analysis
Organic Chemistry
32.5K Views

Performing 1D Thin Layer Chromatography
Organic Chemistry
289.9K Views

Nuclear Magnetic Resonance (NMR) Spectroscopy
Organic Chemistry
248.6K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved