열 크로마토그래피
Overview
출처: 지미 프랑코 박사 연구소 - 메리맥 칼리지
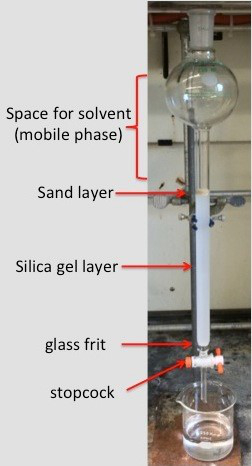
컬럼 크로마토그래피는 화합물을 정화하는 데 가장 유용한 기술 중 하나입니다. 이 기술은 열에 포장된 고정 된 위상과 열을 통과하는 모바일 단계를 활용합니다. 이 기술은 화합물 사이의 극성의 차이를 악용하여 분자가 용이하게 분리 될 수 있게합니다. 1 컬럼 크로마토그래피를 위한 가장 일반적인 고정 단계는 실리카 젤(SiO2)및 알루미나(Al2O3)이며,가장 일반적으로 사용되는 이동상은 유기 용매이다. 2 이동상에 선택된 용매는 정제되는 분자의 극성에 의존한다. 전형적으로 더 많은 극성 화합물은 고정 된 단계를 통해 분자의 통과를 용이하게하기 위해 더 많은 극성 용매를 필요로한다. 정화 공정이 완료되면 절연 된 물질을 산출하기 위해 회전 증발기를 사용하여 수집 된 분획에서 용매를 제거 할 수 있습니다.
Procedure
1. 실리카 젤 슬러리
- 실리카 젤을 에를렌마이어 플라스크에 붓습니다. 포장 재의 무게는 분리되는 샘플의 약 50배여야 합니다. 분리되는 화합물이 매우 유사한 Rf 값을 가지고 있는 경우, 시료당 더 많은 양의 실리카를 사용해야 할 수 있으며, 이는 이 예의 경우이다.
- 에렌마이어 플라스크에 실리카 10g을 배치하면 50 mg의 샘플(플루오레네 45 mg, 테트라페닐포르피린 5 mg)가 분리되고 있기 때문에.
- 용매 시스템 추가(헥산/디클로로메탄, 70%: 30%) 실리카 젤을 함유한 에렌마이어 플라스크에. 모든 실리카 젤이 잘 솔레바닝되도록 충분한 용매를 추가합니다. 실리카는 녹지 않지만, 혼합물은 솔바드될 때 시각적으로 눈에 띄게 될 것입니다. 용매가 추가되면 모든 실리카가 잘 솔레바딩되도록 에를렌마이어 플라스크가 소용돌이치기 위해 날아다닌다.
2. 열 준비
Results
Application and Summary
References
- Mayo, D. W.; Pike, R. M.; Forbes, D. C., Microscale organic laboratory : with multistep and multiscale syntheses. 5th ed.; J. Wiley & Sons: Hoboken, NJ; p xxi, 681 p (2011).
- Armarego, W. L. F.; Chai, C. L. L., Purification of laboratory chemicals. 5th ed.; Butterworth-Heinemann: Amsterdam; Boston; p xv, 609 p (2003).
- Silverman, R. B.; Holladay, M. W., The organic chemistry of drug design and drug action. Third edition / ed.; Elsevier/AP, Academic Press, is an imprint of Elsevier: Amsterdam ; Boston; p xviii, 517 pages (2014).
- Mortensen, D. S.; Perrin-Ninkovic, S. M.; Shevlin, G.; Elsner, J.; Zhao, J.; Whitefield, B. et. al. Optimization of a Series of Triazole Containing Mammalian Target of Rapamycin (mTOR) Kinase Inhibitors and the Discovery of CC-115. Journal of Medicinal Chemistry (2015).
- Davies, D. R.; Johnson, T. M., Isolation of Three Components from Spearmint Oil: An Exercise in Column and Thin-Layer Chromatography. Journal of Chemical Education,84 (2), 318 (2007).
- Taber, D. F.; Hoerrner, R. S., Column chromatography: Isolation of caffeine. Journal of Chemical Education, 68 (1), 73 (1991).
Tags
건너뛰기...
이 컬렉션의 비디오:

Now Playing
열 크로마토그래피
Organic Chemistry
360.1K Views

촉매 소개
Organic Chemistry
34.5K Views

가열된 화학 반응을 위한 역류 시스템의 조립
Organic Chemistry
167.4K Views

실온 이하의 반응 수행
Organic Chemistry
70.6K Views

솔벤트의 슐렌크 라인 전송
Organic Chemistry
41.6K Views

동결 펌프 해동 사이클링으로 액체 를 탈기
Organic Chemistry
56.1K Views

무수성 시약 및 장비 준비
Organic Chemistry
79.3K Views

재결정화로 화합물 정화
Organic Chemistry
708.5K Views

침전을 통한 혼합물의 분리
Organic Chemistry
157.8K Views

고체 액체 추출
Organic Chemistry
237.8K Views

용매제거를 위한 로타리 증발
Organic Chemistry
212.8K Views

분수 증류
Organic Chemistry
334.3K Views

X선 회절 분석을 위한 커지는 결정
Organic Chemistry
32.4K Views

Performing 1D Thin Layer Chromatography
Organic Chemistry
289.7K Views

핵 자기 공명 (NMR) 분광기
Organic Chemistry
247.8K Views
Copyright © 2025 MyJoVE Corporation. 판권 소유