通过评估可逆反应的反应商 (Q) ,可以方便地评估其状态。 对于 m A + n B ⇌ x C + y D 描述的可逆反应,反应商直接从平衡方程 AS 的化学式测定中导出
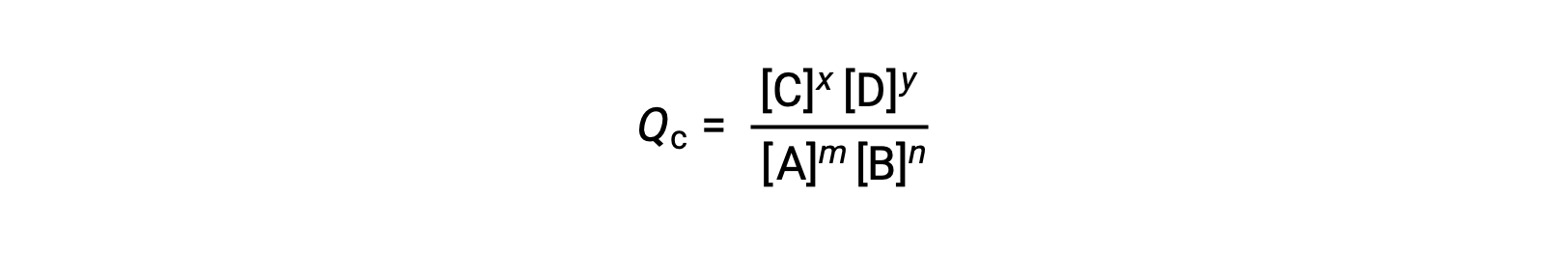
其中,下标 c 表示表达式中使用摩尔浓度。 如果反应物和生成物是气态的,则可以使用分压类似地导出反应商:
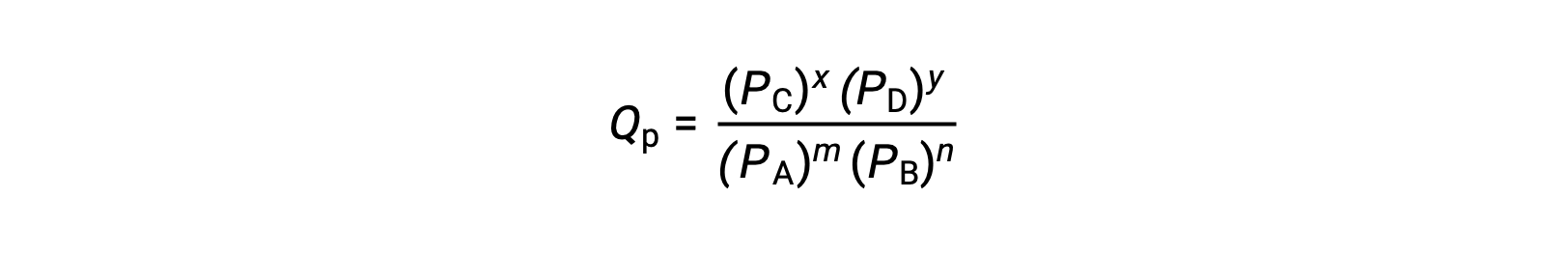
请注意,上述反应商方程式是对更严格的表达式的简化,这些表达式使用相对值表示浓度和压力,而不是绝对值。 这些相对浓度和压力值是无量纲的 (它们没有单位) ;因此,反应商也是如此。
当反应走向平衡时, Q 的数值会有所不同;因此, Q 的数值可以作为反应状态的有用指标。 为了说明这一点,请考虑二氧化硫的氧化:
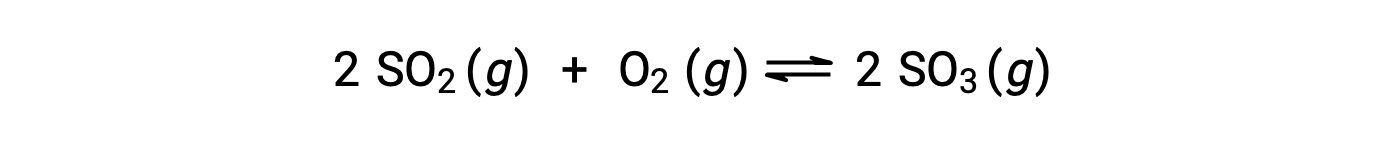
这里可能有两种不同的实验场景,其中一种是由反应物, SO2 和 O2 的混合物引发的,另一种是由生成物, SO3开始的。 对于仅以反应物混合物开始的反应, Q 最初等于零:
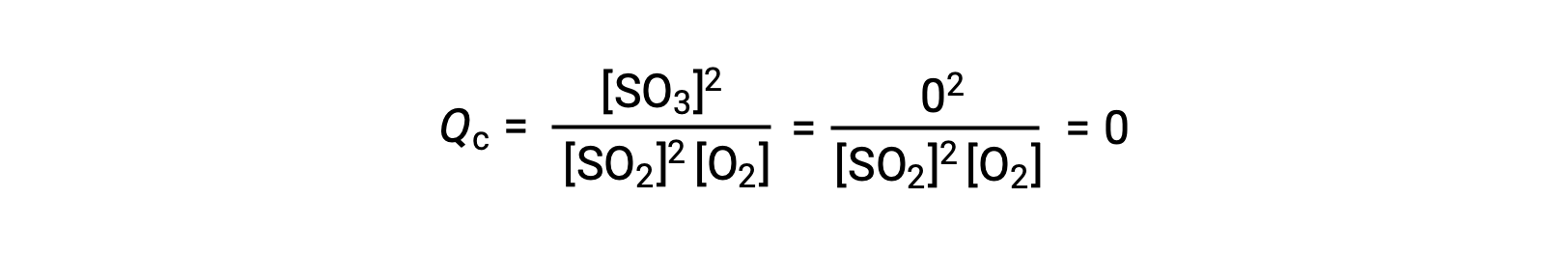
随着反应朝着正向平衡的方向发展,反应物浓度下降 (分母QC也是如此) ,生成物浓度增加 (分子QC也是如此) ,反应商随之增加。 当达到平衡时,反应物和生成物的浓度保持不变,质量控制的值也是如此。
如果反应开始时仅存在生成物,则最初QC未定义值 (不可测量的大或无限) :
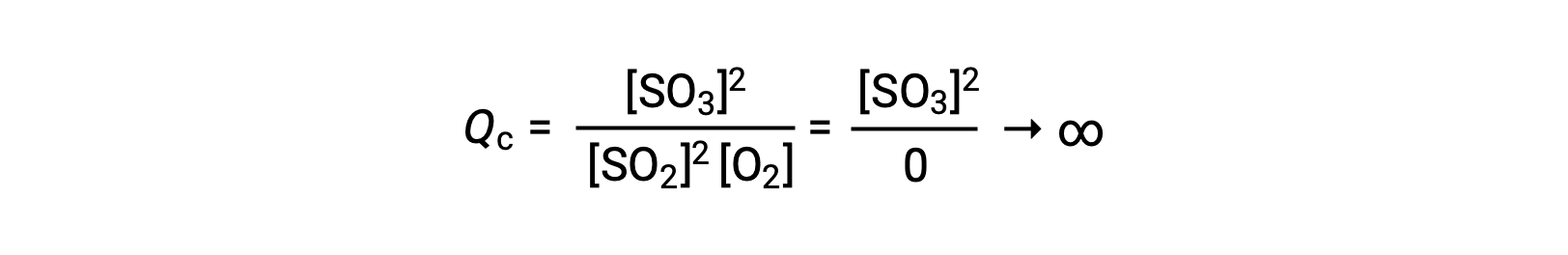
在这种情况下,反应朝着相反方向走向平衡。 生成物浓度和分子 QC随着时间的推移而下降,反应物浓度和分母 Q C增加,反应商随之下降,直到它在平衡状态保持不变。 平衡系统显示的Q的常量值称为平衡常数 K :
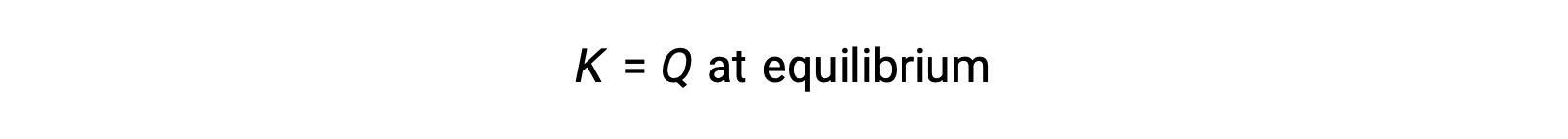
评估反应商
根据该方程:
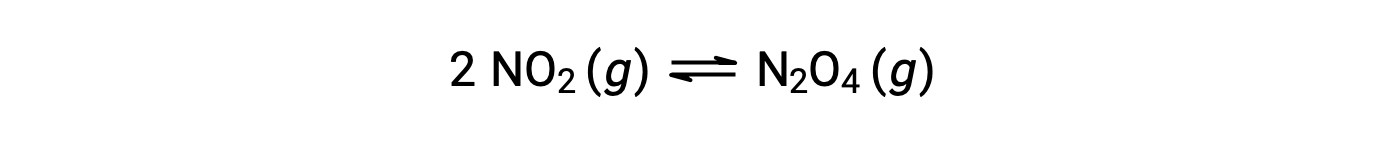
当在 25 °C 条件下将 0.10 mol NO2 添加到 1.0-L 瓶中时,浓度会发生变化,使在平衡状态下, [NO2] = 0.016 M , [N2O4] = 0.042 M。 在形成任何生成物之前, [NO2] = 0.10 M , [N2O4] = 0 M。 因此,
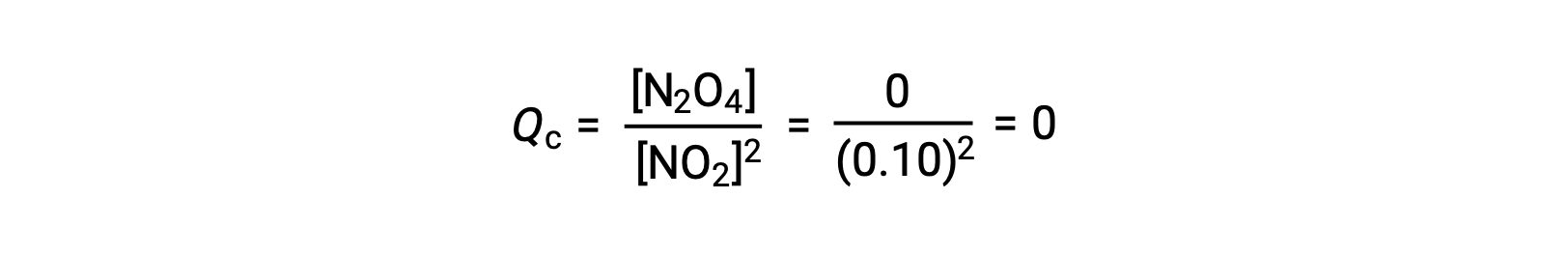
平衡时,
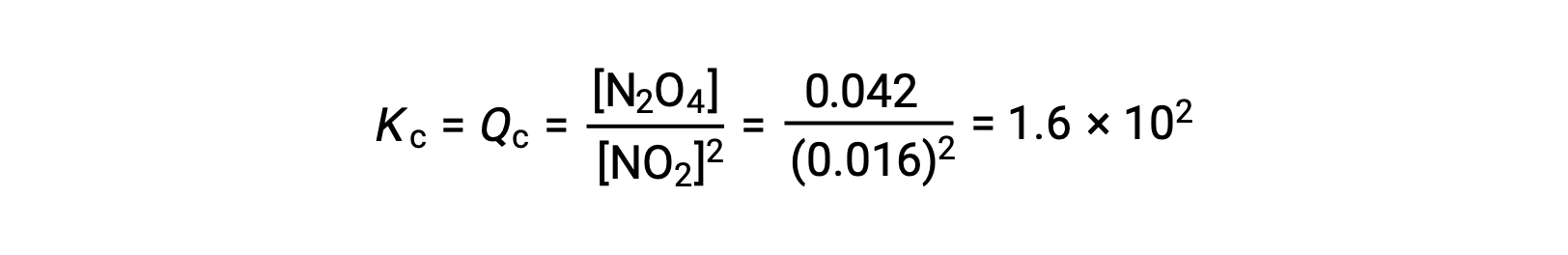
来自章节 14:

Now Playing
14.5 : 反应商
化学平衡
47.1K Views

14.1 : 动态平衡
化学平衡
48.5K Views

14.2 : 平衡常数
化学平衡
45.0K Views

14.3 : 气态反应和非均相反应的平衡
化学平衡
23.2K Views

14.4 : 计算平衡常数
化学平衡
29.7K Views

14.6 : 计算平衡浓度
化学平衡
46.0K Views

14.7 : 勒夏特列原理(Le Chatelier's Principle) :浓度变化
化学平衡
56.3K Views

14.8 : 勒夏特列原理(Le Chatelier's Principle) :体积变化 (压力)
化学平衡
33.2K Views

14.9 : 勒夏特列原理(LeChatelier's Principle) :温度变化
化学平衡
28.2K Views

14.10 : 未知数小x假设
化学平衡
45.3K Views
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。