20.3 : Path Between Thermodynamics States
Consider the two thermodynamic processes involving an ideal gas that are represented by paths AC and ABC in Figure 1:
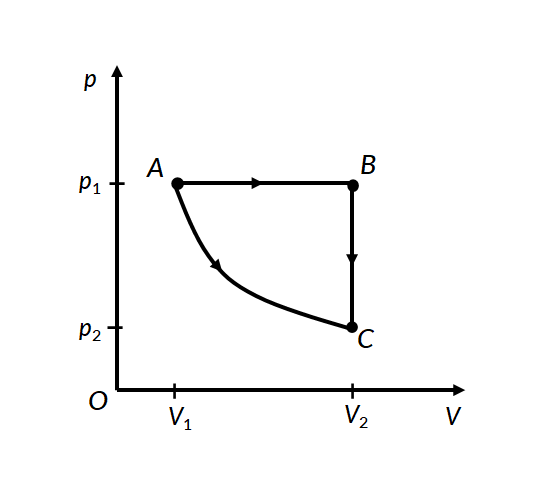
In the first process for path A to C, the gas is kept at constant temperature T. It undergoes an expansion from volume V1 to V2.
The work done by an ideal gas is expressed as
Substituting for pressure as nRT/V from the ideal gas equation and integrating the terms, the work done by an ideal gas at constant temperature is obtained as
In the second process, for path A to B, the ideal gas is first expanded from volume V1 to V2 at constant pressure p1 by applying heat. The gas is then cooled at constant volume V2 along path B to C, such that its pressure drops to p2.
For path A to B, work is done under constant pressure, therefore
For path B to C, since the volume remains constant, no work is done by the gas or on the gas by the surroundings. Therefore the total work done by the gas in this process is the same as the work done for path A to B.
In both processes, the gas expands from volume V1 to volume V2, such that its pressure changes from p1 to p2. However, the work done in both processes is different. This proves that work done by a system is path-dependent.
来自章节 20:

Now Playing
20.3 : Path Between Thermodynamics States
The First Law of Thermodynamics
3.1K Views

20.1 : 热力学系统
The First Law of Thermodynamics
5.0K Views

20.2 : 在体积更改期间完成的工作
The First Law of Thermodynamics
3.9K Views

20.4 : 热和自由膨胀
The First Law of Thermodynamics
1.8K Views

20.5 : 内能
The First Law of Thermodynamics
4.5K Views

20.6 : 热力学第一定律
The First Law of Thermodynamics
4.1K Views

20.7 : 热力学第一定律:解决问题
The First Law of Thermodynamics
2.5K Views

20.8 : 循环进程和孤立系统
The First Law of Thermodynamics
2.7K Views

20.9 : 等温过程
The First Law of Thermodynamics
3.5K Views

20.10 : 等压和等压过程
The First Law of Thermodynamics
3.4K Views

20.11 : 理想气体的热容 I
The First Law of Thermodynamics
2.6K Views

20.12 : 理想气体的热容 II
The First Law of Thermodynamics
2.4K Views

20.13 : 理想气体的热容 III
The First Law of Thermodynamics
2.2K Views

20.14 : 理想气体的绝热过程
The First Law of Thermodynamics
3.0K Views

20.15 : 绝热过程中的压力和体积
The First Law of Thermodynamics
2.6K Views
See More
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。