20.3 : Path Between Thermodynamics States
Consider the two thermodynamic processes involving an ideal gas that are represented by paths AC and ABC in Figure 1:
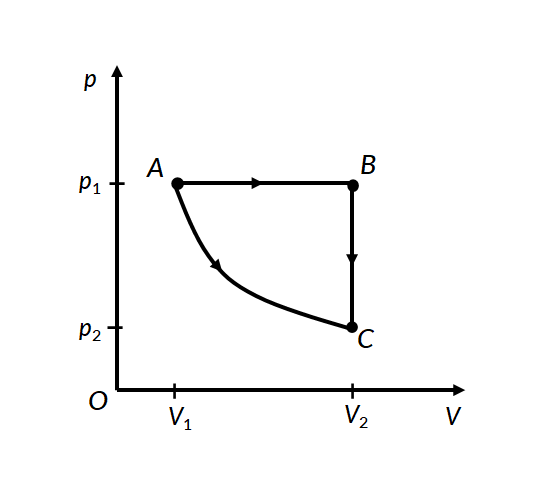
In the first process for path A to C, the gas is kept at constant temperature T. It undergoes an expansion from volume V1 to V2.
The work done by an ideal gas is expressed as
Substituting for pressure as nRT/V from the ideal gas equation and integrating the terms, the work done by an ideal gas at constant temperature is obtained as
In the second process, for path A to B, the ideal gas is first expanded from volume V1 to V2 at constant pressure p1 by applying heat. The gas is then cooled at constant volume V2 along path B to C, such that its pressure drops to p2.
For path A to B, work is done under constant pressure, therefore
For path B to C, since the volume remains constant, no work is done by the gas or on the gas by the surroundings. Therefore the total work done by the gas in this process is the same as the work done for path A to B.
In both processes, the gas expands from volume V1 to volume V2, such that its pressure changes from p1 to p2. However, the work done in both processes is different. This proves that work done by a system is path-dependent.
장에서 20:

Now Playing
20.3 : Path Between Thermodynamics States
The First Law of Thermodynamics
3.0K Views

20.1 : 열역학 시스템
The First Law of Thermodynamics
4.9K Views

20.2 : 볼륨 변경 중 수행된 작업
The First Law of Thermodynamics
3.8K Views

20.4 : 열 및 자유 팽창
The First Law of Thermodynamics
1.7K Views

20.5 : 내부 에너지
The First Law of Thermodynamics
4.3K Views

20.6 : 열역학 제1법칙
The First Law of Thermodynamics
4.0K Views

20.7 : 열역학 제1법칙: 문제 해결
The First Law of Thermodynamics
2.4K Views

20.8 : 순환 프로세스와 고립된 시스템
The First Law of Thermodynamics
2.7K Views

20.9 : 등온 공정
The First Law of Thermodynamics
3.5K Views

20.10 : 등코릭 및 등압 과정
The First Law of Thermodynamics
3.2K Views

20.11 : 이상 기체의 열용량 I
The First Law of Thermodynamics
2.5K Views

20.12 : 이상 기체 II의 열용량
The First Law of Thermodynamics
2.3K Views

20.13 : 이상 기체 III의 열용량
The First Law of Thermodynamics
2.1K Views

20.14 : 이상 기체를 위한 단열 공정
The First Law of Thermodynamics
3.0K Views

20.15 : Pressure and Volume in an Adiabatic Process
The First Law of Thermodynamics
2.6K Views
See More
Copyright © 2025 MyJoVE Corporation. 판권 소유