Method Article
舌下免疫治疗作为一种替代起到保护作用对急性呼吸道感染
摘要
The present work illustrates the convenience of using sublingual immunotherapy to boost the innate immune response in the lungs and confer protection against acute pneumococcal pneumonia in mouse.
摘要
Sublingual route has been widely used to deliver small molecules into the bloodstream and to modulate the immune response at different sites. It has been shown to effectively induce humoral and cellular responses at systemic and mucosal sites, namely the lungs and urogenital tract. Sublingual vaccination can promote protection against infections at the lower and upper respiratory tract; it can also promote tolerance to allergens and ameliorate asthma symptoms. Modulation of lung’s immune response by sublingual immunotherapy (SLIT) is safer than direct administration of formulations by intranasal route because it does not require delivery of potentially harmful molecules directly into the airways. In contrast to intranasal delivery, side effects involving brain toxicity or facial paralysis are not promoted by SLIT. The immune mechanisms underlying SLIT remain elusive and its use for the treatment of acute lung infections has not yet been explored. Thus, development of appropriate animal models of SLIT is needed to further explore its potential advantages.
This work shows how to perform sublingual administration of therapeutic agents in mice to evaluate their ability to protect against acute pneumococcal pneumonia. Technical aspects of mouse handling during sublingual inoculation, precise identification of sublingual mucosa, draining lymph nodes and isolation of tissues, bronchoalveolar lavage and lungs are illustrated. Protocols for single cell suspension preparation for FACS analysis are described in detail. Other downstream applications for the analysis of the immune response are discussed. Technical aspects of the preparation of Streptococcus pneumoniae inoculum and intranasal challenge of mice are also explained.
SLIT is a simple technique that allows screening of candidate molecules to modulate lungs’ immune response. Parameters affecting the success of SLIT are related to molecular size, susceptibility to degradation and stability of highly concentrated formulations.
引言
The overall goal of this work is to illustrate the benefits of sublingual immunotherapy for the treatment of acute respiratory infections (ARI) and present the advantages of this delivery route compared to other routes of administration, namely intranasal.
ARI cause millions of deaths every year especially in children under five. Streptococcus pneumoniae remains as one of the major etiological agents of bacterial pneumonia in infants and the elderly1,2. To present, the main available treatment relies on the use of antibiotics but resistant strains are continuously arising3,4.
SLIT induces broad responses at systemic and also mucosal level, particularly at the respiratory tract5. It has proven effectiveness against influenza infection, promoting long term protection with production of humoral and cellular responses6,7. Besides, it has been shown that prophylactic treatment with bacterial lysates delivered by sublingual route reduced exacerbations of chronic obstructive bronchitis in the elderly8 and prevented recurrent respiratory infections in children9. SLIT has been widely used for the treatment of allergies and asthma. Clinical studies had not only demonstrated its efficacy to modulate the immune response in the respiratory tract but also its safety10. Despite the growing interest of pharmaceutical companies and researchers in SLIT, the mechanisms involved in the induction of mucosal immune responses after sublingual delivery of compounds remain obscure. Recently, attention has been focused on the mechanisms promoting tolerance associated with allergen desensitization. It has been proposed that resident and recruited cells at the sublingual mucosa, like dendritic cells and macrophages, can promote tolerance after SLIT11-13. Dendritic cells of the oral mucosa can promote IFN-gamma and IL-10 producing T helper cells11 as well as recirculate to the distal genital mucosa and promote CD8+ T cells14. However, little is known about the impact of SLIT on innate cells or its capacity to improve pathogen clearance during acute respiratory infections.
The natural control of pneumococcal infection in the lungs greatly depends on the efficient and swift activation of local innate defences. We previously showed that enhancement of lungs’ innate immunity by a single intranasal dose of flagellin (FliC), a TLR5 and NLRC4 agonist, protects 75-100% of mice challenged with a lethal dose of a clinical isolate of Streptococcus pneumoniae serotype 1. This protection was shown to be dependent on local recruitment of GR1+ cells (likely polymorphonuclear neutrophils, PMNs) and not dependent on antibodies, B or T cells15.
Flagellin is the structural component of the bacterial flagellum. In its monomeric form it is recognized by two Pathogen Recognition Receptors (PRRs), TLR5 that senses extracellular FliC16 and NLRC4/NAIP5 inflammasome that detects intracellular flagellin17,18. When FliC is sensed by the PRRs an important inflammatory response is triggered. We and others have demonstrated that instillation of purified FliC from Salmonella enterica serovar Typhimurium into the lungs drives swift production of chemokines and cytokines specially when recognized by the lungs’ epithelium that in turn orchestrate the recruitment of immune cells into the airways, mainly PMNs15,19-21. Although transient, the substantial neutrophil infiltration that takes place into the airways after nasal delivery of FliC could be a concern if moving towards clinical therapies for human use. Excessive inflammation could be detrimental for the lungs’ function. Moreover, it has been shown that intranasal delivery of immunostimulatory molecules may cause facial paralysis and/or brain toxicity22-24.
Sublingual immunotherapy offers a safer alternative to modulate the immune response in the respiratory tract compared to the intranasal route. It is non-invasive, painless, simple and has good patient compliance25. Furthermore, as mentioned before, it can induce protective responses in the respiratory mucosa without the risks associated to direct intranasal or intrapulmonary delivery of formulations. Sublingual route could be alternatively used to deliver molecules that have great effects onto the lung’s immune system but that have been proven to be toxic or to elicit great inflammation when administered intranasally. Besides these advantages, formulations for sublingual immunotherapy have lower cost of manufacture since non-sterile products can be delivered by this route and endotoxic shock is not a concern for SLIT. On the other hand, it is worth noticing that higher doses of the immunostimulatory compounds compared to those used by intranasal or parenteral routes are necessary to induce an immune response in the lungs; also highly concentrated solutions are needed when using the mouse model of SLIT since the anatomical site where the formulations are deposited is small.
Based on our previous published data, we developed a model of protection using sublingual immunotherapy with flagellin as model immunostimulant. We demonstrated that a single dose of flagellin induced 60% survival against invasive pneumococcal pneumonia caused by the serotype 1 strain while all mice in the control group died of infection within 5 days. Flow cytometry analysis showed that higher numbers of PMN are recruited into the airways of protected animals after sublingual treatment with flagellin suggesting that these cells might be involved in the mechanism of protection induced by sublingual immunotherapy.
This video shows in detail how to perform sublingual immunotherapy and also how to recover relevant tissue from the sublingual mucosa, draining lymph nodes as well as lungs and airways to perform further analysis. Additionally, it illustrates the general technique of cell preparation for FACS analysis and briefly shows how to prepare Streptococcus pneumoniae suspensions and how to perform intranasal infections in mouse to set up the acute infection model.
研究方案
乌拉圭 - 涉及动物的过程中按照协议车次071140-000821-12和08052010批准的名誉委员会,动物实验和医学院,大学德拉共和的指令董事会执行。
治疗剂的1舌下给药
- 制备含有治疗剂的溶液来进行试验。调整浓度来管理的每只小鼠10微升的最大音量。
注:从沙门纯化鼠伤寒沙门氏菌鞭毛蛋白的最佳剂量,以起到保护作用在小鼠感染的第一致死量S。肺炎链球菌血清型1 E1586,造成100%的死亡率为10微克/鼠标。鞭毛蛋白溶液必须在65℃下加热5分钟,以确保单体的释放。有关鞭毛蛋白的纯化更多信息,请参考26。- VAR不同的免疫调节剂的,根据其分子大小,纯度,易受蛋白酶解和使用粘膜粘附剂ÿ有效浓度。调整最佳浓度为每一种化合物进行测试,以最大限度地发挥其效果。如果先前的研究鼻内已经进行了特定的化合物,使用起始剂量5〜10倍,通过舌下含化路线,以测试其有效性。
- 通过注入含110毫克/公斤氯胺酮5.5毫克/千克Xylacine鸡尾酒麻醉的老鼠,让动物休息7〜10分钟。
- 通过轻轻按压后腿之一的足垫确认正确anaesthetization;如果适当麻醉的动物不会响应于刺激移动。
- 撒一层薄薄的兽医药膏在每个鼠标来防止干燥,同时在麻醉状态下的眼睛。
注:Inhalatory麻醉药等异氟烷,也可以使用代替氯胺酮/ Xylacine如果系统装备PED与感应腔和鼻锥是可用的。使用感应室anaesthetise动物和管理的免疫刺激舌下途径。紧接在动物连接到鼻锥至少15分钟,以保持它在麻醉状态下,避免吞咽并允许治疗性化合物的吸收。 - 吸取含有免疫刺激剂或赋形剂对照溶液中的溶液;用拇指和食指的非优势手取小鼠并保持在垂直位置。
- 使用优势手位置的一对闭合钳子舌下和使用中指和无名指保持在适当位置,稍微打开钳子抬起舌。
- 取吸管和管理该溶液到舌头的嘴和背侧的楼层。
- 除去钳子,让把它放回笼子前鼠标其余为3〜5分钟。以确保正常体温保持在麻醉麦克风E,连接笼一笼加热器系统。如果这样的系统不可用时,处小鼠属于同一治疗组放回相应的笼一个接一个地彼此在床上用品和部分地覆盖它们与清洁薄纸片,以帮助他们维持体温。
- 收集组织样本的免疫调节剂,滴眼后的任何时间点的变化来分析诱导治疗的细胞群。
注意:在鞭毛这个特定的协议管理,进行2小时的挑战面前。确定治疗和挑战针对每一特定治疗剂和病原体之间的最佳时间,以进行测试。
2,菌悬液的制备和鼻内的挑战与肺炎链球菌
注:S。肺炎是一种自然的人类病原体,可能会导致危及生命的疾病,如侵袭性肺炎,败血症和脑膜炎。吸入或与粘膜接触的时候,可能会出现传输。因此,可能已经在接触与S的所有样品肺炎必须使用二类生物安全柜适当的生物安全二级机构进行处理。检查机构对处理第二类病原体的防护服,废物处理和额外的保安措施可能适用的标准操作程序。受感染的动物应存放在单独的通风笼中装有HEPA过滤器隔离。抗肺炎球菌疫苗和抗生素治疗是可用的。欲了解更多信息,请参阅参考资料27和1。
- 解冻的工作股票停牌肺炎链球菌作为15所述的方法制备已知的细菌菌落形成单位数等分。
- 离心5分钟在2500 XG和RT。
- 弃上清,通过暂停其在1毫升圣洗菌体erile生理盐水溶液。准备菌悬液,稀释或动物的挑战时,使用过滤嘴。
- 在步骤2.2离心机再次描述。
- 弃去上清,重悬沉淀在无菌盐水中的适当体积,以获得4×10 5 CFU / 50微升的悬浮液。该剂量对应于最小的细菌剂量的S。肺炎链球菌血清型1 E1586导致100%的死亡率BALB / c小鼠,根据以往的研究15。
注:在建立小鼠肺炎球菌肺炎模型,最小剂量的细菌引起的死亡率为100%,必须为每个特定的细菌菌株血清型和小鼠品系的组合来决定。 - 通过涡旋或移液器上下吹吸5次均匀的菌悬液。
- 负载50微升的菌悬液用无菌过滤嘴和灌输的总体积到麻醉小鼠的鼻孔。按住鼠标upriGHT 2分钟,让它休息背姿势2分钟以上。适用于兽医药膏到眼睛,回到动物的笼子;一定要保持正常体温,而在麻醉下。
请注意:在本研究中的细菌的挑战是在50微升体积中进行,以确保输送的至少90%的总CFU进入肺部如15,28预先确定的。为了尽量减少对动物的较小体积( 例如 20微升)可用于窘迫。然而,有效地提供细菌进入肺部,必须检查;这可以通过收获肺5分钟挑战后和通过电镀系列稀释到血琼脂平板上在肺部的匀浆计数菌落形成单位来进行。 - 通过电镀连续10倍稀释到血琼脂平板上确认在细菌悬浮液用于感染的CFU数。孵育在o / n 37℃,5%CO2和计数粘液菌落呈现人的绿色光环特征数PHA溶血性细菌。
3,组织收集和样品制备用于流式细胞术(FACS)分析
3.1)组织收集
- 安乐死的动物通过颈椎脱位或使用CO 2室;打开胸腔,一路到颈部并沿着前腿切开颈部及颌下区的腹侧暴露。
- 与精尖弯血管钳轻轻拉起唾液腺及邻近软组织暴露的楼口的背侧。采用弧形薄尖镊子,把下颌骨和下颌配件淋巴结轻轻拉起,并将其放置在含有完全RPMI(cRPMI管,500 ML-10%胎牛血清,5ml含万单位/毫升的溶液根据下游过程,将青霉素和10毫克/毫升链霉素溶液和5ml L-谷氨酰胺的200mM)或核酸防腐剂溶液在后面进行。
- 以打开胸腔使在隔膜中的切口;使用一对鼠齿钳钳胸骨剑突软骨和精心切割的假排骨一路上涨开始,直到达到的地步,真正满足肋骨胸骨柄的肋骨在两个背两侧。
- 通过举办与钳胸骨剑突软骨,拉起轻轻地暴露胸腔的器官。
- 完全通过切割所述第一肋与锁骨除去肋。胸腺将出现的两个波瓣位于胸部靠近心脏的基部的前腹侧部分的白色组织。
- 就拿叶一通过用钳子夹紧,并用剪刀去掉它的下表面和心包之间的韧带。进行删除第二个肺叶。
- 确定腹腔并通过沿米的中间轴线切割打开它uscular墙揭露器官。用钳子剪断后腔静脉和主动脉;除去过量的血液与吸收薄纸。
- 分析肺泡的居民,浸润细胞群进行支气管肺泡灌洗(BAL)。切开的肌肉在颈部,露出气管和食道的腹侧部;将它们分开的结构,外侧和足背两侧作切口。
- 提起用钳子气管和做一个小切口,用手术刀引进薄尖移液管充填用1毫升的PBS不含Ca 2 + / Mg 2 +的加1毫米EDTA。灌输和吸出的总体积的至少三倍;吸液和细胞悬浮液转移到一个无菌的1.5mL管中,并放置在冰上。
- 分析存在于肺实质的细胞群,第一灌注肺通过注射5毫升的PBS不含Ca 2 + / Mg 2 +的加1mM EDTA中进入右心室的心脏。
注意:这将消除大部分的红血细胞和免疫细胞的存在入肺'的血管。如果正确进行灌注,肺的颜色会由粉红转为白色。 - 从左心室基部夹紧它隔离肺心脏和精心切割的血管用剪刀将其完全移除。采取灌注肺,并放置在cRPMI还是取决于下游分析核酸防腐液来进行。
- 在舌下粘膜的细胞群的分析,分离出动物的头部和移除唾液腺和邻近软组织,如果它尚未在步骤3.2.1完成。
- 使在口的每一侧上的切口,直至到达下颌骨关节和分离劣颚连同口中的舌和地板上,用销固定其上的夹层板。拉舌;用手术刀做一个切口,其中巴舌本身满足口底直到到达第三臼齿,以暴露舌下粘膜。
- 彻底去除舌头;取0.5mm的穿刺打孔,并把它旁边的下门牙。从舌下组织和媒体的牙龈切开插入轻轻口的地板已经被切出完全。
- 重复一次,现在把活检穿孔接近第三磨牙,以彻底清除舌下组织。放在一个干净的试管含cRPMI或核酸防腐剂。
3.2)的流式细胞仪分析的样品制备。
- 转移肺'组织,从各小鼠中分离到24孔板上,并用干净的剪刀直至获得小块的大约2mm的组织剁碎它们。加入含30mg II型胶原酶,50微克DNA酶-I的1ml RPMI无FBS的消化培养基为1毫升每孔。移液器上下五次孵育37℃和5%CO 2的40分钟。
- 对于细胞群在舌下组织分析,替代消化介质3.2.1具有一个含2台分散酶,30毫克II型胶原酶,50微克DNA酶-I在1毫升的RPMI。孵育来自一只小鼠在500μl消化培养基的20分钟收集的组织在37℃下在轨道摇床在50rpm。
- 孵育后,吸移管上下高达10倍或30秒,直到大部分组织已被破坏。过滤细胞悬液,虽然有40微米的无菌细胞过滤和洗涤用5毫升的PBS添加5毫米EDTA。
注:细胞外基质和纤维组织的完全消化将无法实现。然而,在胶原酶和/或分散酶或侵略性研磨的存在较长的温育时间,不推荐,因为它会导致增加的细胞死亡和破坏影响的F的整体结果胞外蛋白ACS的分析。 - 离心400 XG,5分钟,4℃。
- 对BAL中的细胞群的分析,离心细胞,在400 XG,5分钟,4℃,并继续到步骤3.2.4。
- 在淋巴结细胞群的分析,放置在无菌培养皿70微米的细胞过滤网,把淋巴结一起用1ml cRPMI进入过滤器。取出2毫升无菌注射器的暴跌,并把它作为一个杵粉碎对滤网的网孔的淋巴结。冲洗的细胞过滤网用1ml新鲜cRPMI和从培养皿转移细胞到无菌试管中。
- 取每个样品的代表性部分,并用台盼蓝染色它来确定活细胞数目。
- 将细胞重悬于FACS-EDTA:PBS-5mM的EDTA-1%牛血清白蛋白,以弥补为2×10 7个细胞/ ml的悬浮液,并添加50μl的成仪管。
- 准备包含进近2倍的抗体组合抗体可以根据可用的FACS仪器表面标记和荧光染料的opriate组合。加入50微升2倍抗体混合液到含有细胞悬浮液各管中。
注:滴定各荧光物质标记的抗体,以确定最佳的量被使用,一个详细的协议见参考文献29。 - 在冰上孵育在黑暗中30分钟。
- 用3ml FACS-EDTA洗一次,并在400 XG在4℃下离心5分钟,降速的细胞,使细胞重新悬浮于200μl的相同缓冲液中,并在流式细胞仪分析。
注:如果处理样本的大数目,可在U形底96孔板中,而不是细胞仪试管中进行的染色方案为如上所述的FACS分析。然而,如果使用96孔板的洗涤步骤,必须通过加入到200μl的FACS-EDTA的和重复它4次停转的细胞以400×g离心5分钟,在4℃下每鹫之间进行吴步。 - 此时,固定的样本进行分析的流式细胞仪后(最多72小时后固定)。
- 以固定细胞,用流式细胞仪抗体标记后在PBS洗没有钙离子/镁离子 ,1毫米EDTA无FBS的细胞。使细胞悬浮在50μl的相同缓冲液中和在高渗(2X)的PBS加入50微升新鲜制备的4%多聚甲醛溶液中没有的Ca 2 + / Mg 2 +的 。
- 孵育20分钟,在室温,在FACS-EDTA洗3次。
- 将细胞重悬于200μl的FACS-EDTA,并储存于4℃,并从光保护长达72小时。
注:FSC-SSC会受到固定。如果固定的样品检查与制造商的荧光标记抗体的兼容性,因为串联染料可在固定剂剂存在下被降解。如果样本源自受感染的动物固定强烈建议,以确保没有可行的病原体会出现analy时唱流式细胞仪机样,因为可以采集样品的过程中产生的microaerosols。
4,总RNA提取,cDNA合成和核酸荧光PCR检测。
4.1)RNA提取和cDNA合成。
- 均质化中的选择由机械破碎的核酸防腐剂溶液的组织( 例如,使用转子-定子匀化器,高速摇动组织ruptor和珠子等)。离心机以12,600 xg离心15分钟,4℃以除去组织碎片。将上清转移到一个干净的试管中。
- 提取与所选择的以下制造商的指示的方法中的RNA。
注:RNA是非常容易分解,如果不打算使用直线距离隔离后,使等分试样,并将其在无RNA酶的管储存于-80℃。避免反复冻融。管子必须用手套在任何时候都被处理。解冻的样本后,lways让他们在冰上。 - 测核酸的260nm处的吸光度,并计算在微克/微升的浓度。
- 准备DNA酶-I混合加入(1样本):7.6μL超纯水,1微升10X DNA酶-I缓冲液,0.4μLDNA酶-I(放大级)股票1单位/微升,并加入8.4微升DNA酶-I混含1微克总RNA的每个样本。
- 使用的RNA在1微克/微升的浓度和加入1微升的总RNA作为模板进行逆转录反应(RT-PCR)。如果样本是太稀的浓度是低于预期的,添加更大的总RNA,而不是水的体积。加入特别是如果所选择的RNA提取协议涉及苯酚 - 氯仿混合物自苯酚痕迹可能影响RT-PCR方法的收率的RNA时,不超过最终反应体积的20%。
- 孵育15分钟,在室温下,接着用10分钟,在4℃下或者我CE。 (不要超过孵育时间!)
- 加入1μlEDTA的25mM(分子生物学级),每管和孵育在65℃下进行10分钟以灭活DNA酶-I。
- 制备逆转录(RT)混合物如下(1反应):1μl的随机六聚体引物的库存0.2毫克/毫升,加入1μl的dNTP股票的10mM,加入4μl5X M-MLV-RT缓冲液,2微升的DTT 0.1M,1微升RNA酶缺货40U /微升,和1微升的M-MLV retrotranscriptase股票200 U /μL。
- 加入10微升的RT-PCR的混合的10微升DNA酶I反应管。
- 进行中根据以下程序热循环仪进行PCR反应:
1X周期:10分钟,25℃; 50分钟,37℃; 15分钟,70℃ - 通过加入80μl的超纯水5:稀释的cDNA 1。储存于-20℃。
4.2),实时荧光定量PCR(定量PCR)。
- 准备定量PCR反应混合物如下:(1反应):含有Taq酶P 5μl反应混合液olymerase,SYBR Green染料,PCR缓冲液,dNTP混合物和氯化镁 (见4.2.2下文); 0.9微升正向引物10μM的储备液,0.9微升的10μM的储备液的反向引物,1.2微升的超纯水,和2μlcDNA模板预先稀释如步骤4.1.10所示。
注:本部分所使用试剂浓度和循环方案进行了优化,要进行专门的"表材料与试剂"中描述的试剂和仪器,其他品牌可以使用,但反应体积,反应物浓度和骑自行车的协议可能会有所不同。进行RT-qPCR的之前,请检查您的制造商的说明。 - 设置了定量PCR仪,如下所示:
1X周期:15分钟,95℃
40X周期:15秒,95℃,接着1分钟,60℃(在这一点上获取荧光)。
注:对于mRNA的相对定量根据Ct法30 referencE基因必须被选择为Ct值的归一化。选择的参照基因应该具体分析条件下测试其表达可能会有所不同; Actb的,GAPDH或18S是一些基因通常选择作为参考。 - 设置阈值,并分析数据。
结果
舌下免疫治疗可以成功地用于调节肺部的免疫应答。我们发现,鞭毛蛋白,该TLR5和NLRC4激动剂的单一剂量,可诱导的mRNA的显著上调编码趋化因子CXCL1,CCL20和细胞因子IL-6相比盐水处理的对照组。折叠诱导的mRNA水平的缝后达到峰值8小时和20小时( 图1),之后返回到基础水平。然而,当缝进行2小时前鼻内感染带S。肺炎,CXCL1和IL6的mRNA水平保持显著缝后相比,未经处理的动物( 图2)上调甚至24小时。
BAL中的细胞群体和肺组织中通过FACS的分析表明,与FLIC通过舌下途径治疗的动物在肺'组织( 图3)增加了中性粒细胞的数目在呼吸道,但并非如此。
最后,肺炎球菌攻击后生存先前的Flic治疗舌下路线或用生理盐水作为对照组动物进行了比较。如示于图4中 ,缝有鞭毛促进保护和增加的存活对急性肺炎球菌性肺炎。
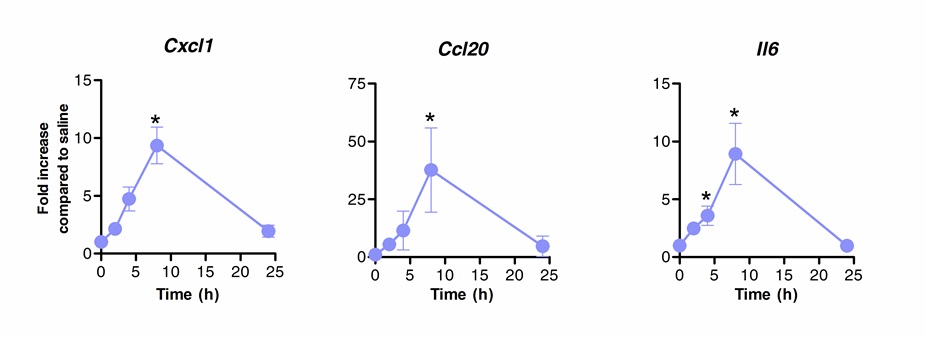
图1动力学肺'转录信息的舌下免疫与鞭毛蛋白后,将8至10周龄的BALB / c小鼠(n = 4)与10微克鞭毛蛋白或盐水的麻醉下舌下途径处理。肺部收集在不同的时间点,并放置在核酸防腐剂。总RNA的提取,进行与合成cDNA。 mRNA水平通过使用TABL上市的特异性引物实时PCR评价ê1。相对定量是根据使用Actb的 mRNA水平正常化ΔCT方法进行。相比于盐水治疗组的平均±SEM结果显示为倍的增加。星号表示,根据Mann-Whitney检验计算统计学显著差异(P <0.05)。结果代表2个独立的实验。
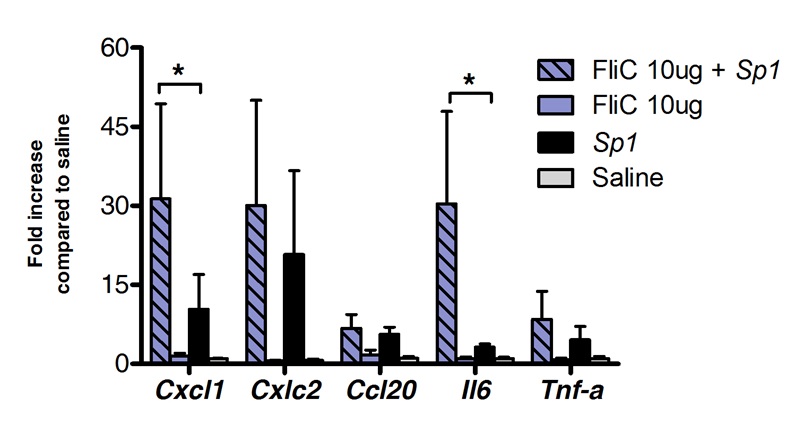
图2肺'舌下免疫与 (为对照组为治疗组N = 4和n = 7) 的鞭毛蛋白。8到10周龄的BALB / c小鼠后,肺炎球菌肺炎中的转录概况由具有10鞭毛蛋白或盐水微克处理在麻醉下舌下途径。 2小时后小鼠鼻内用最小致死量(MLD),造成10挑战S的 临床分离株的0%死亡率肺炎链球菌血清型1 E1585,相当于4×10 5 CFU / 50微升。肺部收集在攻击后24小时,并储存在核酸防腐剂,直到RNA提取和cDNA合成进行。实时进行PCR(参见引物列表在表1中)和相对定量是根据使用Actb的 mRNA水平进行归一化ΔCT法进行的。相比于盐水治疗组的平均±SEM结果显示为倍的增加。星号表示,根据Mann-Whitney检验计算统计学显著差异(P <0.05)。
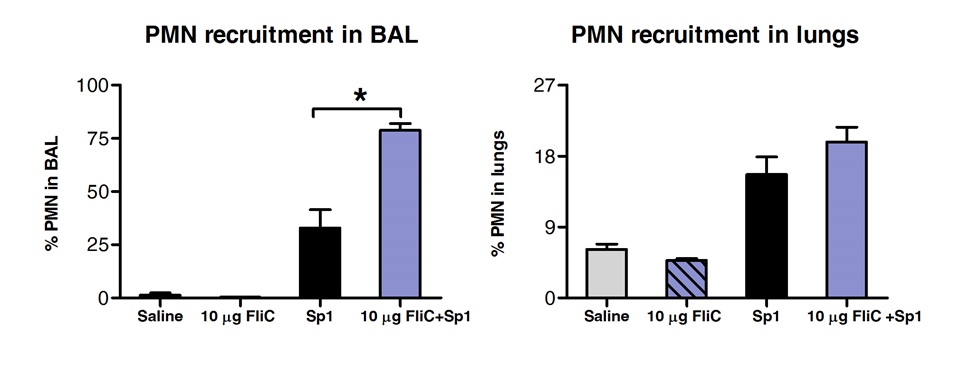
中性粒细胞的图3分析(PMN)招聘狭缝后肺部的组织和呼吸道。八至10周龄的BALB / c小鼠(n = 4)与10微克鞭毛蛋白或盐水的麻醉下舌下途径处理。 2小时后小鼠鼻内用S的MLD挑战肺炎链球菌血清型1 E1585。在攻击后24小时,BAL进行和肺进行处理,用于FACS分析。中性粒细胞鉴定为Ly6G 高 / CD11b的高 / CD11c的阴性细胞和基于FCS的SSC的信息。结果表示为中性粒细胞的相对的BAL或肺细胞总数的百分比。棒代表平均±标准差。星号表示根据单程Mann-Whitney检验计算统计学显著差异(p <0.05)。
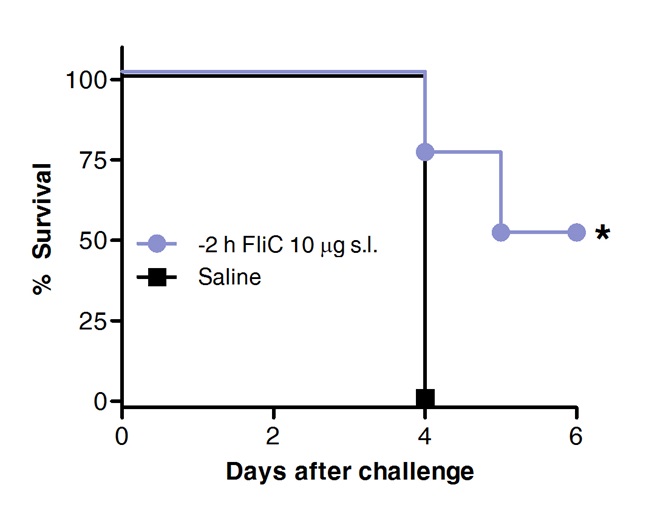
图4。缝与鞭毛蛋白保护小鼠免于急性肺炎球菌性肺炎。 8到10周龄的BALB / c小鼠(n = 8)用10微克鞭毛蛋白或盐水的麻醉下处理通过舌下途径。 2小时后小鼠鼻内用S的MLD挑战肺炎链球菌血清型1 E1585。生存评估每天。根据对数秩(曼特尔 - 考克斯)测试Kaplan-Meier曲线进行了比较。星号表示统计显著差异(P <0.05)。结果代表了2个独立的实验。
| 产品名称 | 序列5'-3' | PCR产物的长度(bp)的 |
| MB-actin_F | GCTTCTTTGCAGCTCCTTCGT | 68 |
| MB-actin_R | CGTCATCCATGGCGAACTG | |
| mCCL20_F | TTTTGGGATGGAATTGGACAC | 69 |
| mCCL20_R | TGCAGGTGAAGCCTTCAACC | |
| mCXCL1_F | CTTGGTTCAGAAAATTGTCCAAAA | 84 |
| mCXCL1_R | ACGGTGCCATCAGAGCAGTCT | |
| MIL-6_F | GTTCTCTGGGAAATCGTGGAAA | 78 |
| MIL-6_R | AAGTGCATCATCGTTGTTCATACA | |
| mTNFalpha_F | CATCTTCTCAAAATTCGAGTGACAA | 63 |
| mTNFalpha_R | CCTCCACTTGGTGGTTTGCT | |
| mCxcl2_F | CCCTCAACGGAAGAACCAAA | 72 |
| mCxcl2_R | CACATCAGGTACGATCCAGGC |
用于实时PCR分析表1中的引物列表。用于定量PCR分析的具体的引物序列。正向和反向引物对小鼠ACTB,Cccl20,CXCL1,IL6,肿瘤坏死因子和CXCL1表示为5'-3'序列与预期产物长度为碱基对(bp)。
讨论
治疗剂舌下给药已被证实为调制在呼吸道的免疫应答的有用手段。狭缝的用于呼吸病症的治疗的主要优点是,它不涉及直接递送化合物进入肺部或鼻腔的,比基于鼻内给药31治疗更安全。
舌下免疫可用于调制方式不同的免疫反应,或者用于诱导的调控反应,可以改善变应性炎症和哮喘32症状或诱导的先天免疫机制瞬时激活治疗急性肺部感染,如下所示。
在这个视频呈现的小鼠模型是不同的化合物作为治疗剂用于狭缝的筛选的方便方法。
这种动物模型提供了一个有用的方法来确定的影响缝在肺部的免疫应答以及在其他器官( 例如 ,引流淋巴结或远端粘膜部位),可以不通过使用体外模型来模拟。虽然有使用说明舌下免疫治疗获得效果了多篇论文,并没有做出详细的方法舌下给药的方法可用呢。此外,该模型可用于舌下疫苗旨在在呼吸道赋予全身以及局部保护评价。
如图所示的附带视频,化合物的舌下给药是,可以在没有大量的训练,需要能够容易地进行一个简单的过程。通常情况下,一个人精通动物处理,需要1小时,在一组使用注射麻醉剂如本协议中所述的10只小鼠进行缝。如果肺炎球菌的挑战进行为好,另外的90分钟将被要求准备细菌悬浮液,并执行这些动物的鼻内攻击。
这里介绍的FACS协议允许该狭缝中的给药本地站点冲击的便利特性,排在肺部的细胞动力学淋巴结以及它们的效果。
支气管含量和肺实质的单独的分析是非常重要的,以鉴别呼吸道的免疫居民和从那些留在组织内的浸润细胞类型。在BAL内容的分析使肺泡巨噬细胞周转的研究以及细胞招募的动态感应到不同的治疗肺泡腔, 如 ,中性粒细胞,嗜酸性粒细胞,单核细胞。 BAL也可以使用酶联免疫吸附测定(ELISA)或检测舌下疫苗接种后引起分泌型IgA抗体,以评估所分泌的细胞因子和趋化因子的存在下进行。研究肺"组织将允许其它细胞类型,古典的树突状细胞,T细胞和B细胞的表征。
BAL样本和淋巴结的流式细胞仪分析制备简单。样品采集后,通常60分钟必须完成的染色方案10-20样本。与此相反,从肺或舌下组织细胞的分离将需要更多的时间,因为需要细胞外基质的消化。通过舌下途径递送的治疗剂的吸收可以通过使用体内成像系统,荧光或放射性标记的分子的跟踪处理。
舌下免疫是一个有吸引力的方法,以有效地诱导在呼吸道的免疫反应以及全身性,可用于治疗或预防呼吸病症。确定激活与缝后我在呼吸道的免疫反应的耐受机制的阐明š关键允许的,可以单独使用或组合使用以针对不同的呼吸病症可利用的治疗的新治疗策略的合理设计。
披露声明
The authors have nothing to disclose.
致谢
We acknowledge Dr. Jean-Claude Sirard from the Center for Infection and Immunity of Lille, Institute Pasteur de Lille-France, for kindly providing the purified flagellin and Dr. Teresa Camou, Director of the National Reference Laboratory, Ministry of Health of Uruguay for kindly providing the pneumococcal strain.
The authors would like to express their acknowledgement to Mr. Diego Acosta and Mr. Ignacio Turel form BichoFeo Producciones-Uruguay for their commitment and hard work during the entire video production and edition.
This work was supported by the grants PR_FCE_2009_1_2783 and BE_POS_2010_1_2544 from the National Agency of Research and Innovation, ANII from Uruguay, the Program for Development of Basic Sciences, PEDECIBA of Uruguay and Sectoral Commission of Scientific research, CSIC-Universidad de la República, Uruguay.
材料
| Name | Company | Catalog Number | Comments |
| Ketamine solution (50 mg/ml) | Pharma Service, Uruguay | ||
| Xilacine solution (2 %) | Portinco S.A., Uruguay | ||
| Sterile 1 ml syringe | Modern, Uruguay | ||
| Sterile 27 G needle | Modern, Uruguay | ||
| RPMI 1640 | General Electric Health Care | E15885 | |
| Fetal Bovine Serum | ATCC | 302020 | |
| Penicillin/Streptomycin Solution | SIGMA | P4333 | |
| Sterile PBS without Ca2+/Mg2+ | PAA | H21002 | |
| Type-I Collagenase | Life Technologies/Gibco | 17100017 | |
| Deoxyribonuclease I (DNAse-I) | SIGMA | D4513 | |
| Dispase | Life Technologies/Gibco | 17105041 | |
| PerCP-Cy5.5 conjugated rat anti mouse IgG2b anti CD11b | BD | 550993 | Clone M1/70 |
| APC conjugated hamster anti mouse IgG1 anti CD11c | BD | 550261 | Clone HL3 |
| APC-Cy7 conjugated rat anti mouse IgG2a anti Ly6G | BD | 560600 | Clone 1A8 |
| Sterile Saline Solution | Laboratorio Farmaco Uruguayo, Uruguay | ||
| Tryptic Soy Agar | BD Difco, France | 236950 | |
| Defibrinated Sheep Blood | Biokey, Uruguay | ||
| Sterile Petri Dishes | Greiner | 633180 | |
| p10 Pipette | Gilson | F144802 | |
| p20 Pipette | Eppendorf | 3120000097 | |
| p200 Pipette | Gilson | F123601 | |
| p200 Pipette | Capp | C200 | |
| p200 Pipette | Eppendorf | 3120000054 | |
| p1000 Pipette | Eppendorf | 3120000062 | |
| Sterile Filter Tips p10 | Greiner | 771288 | |
| Sterile Filter Tips p200 | Greiner | 739288 | |
| Sterile Filter Tips p1000 | Greiner | 750288 | |
| Vortex | BIOSAN | V1-plus | |
| Stainless steel fine tip forceps | SIGMA | Z168785/Z168777 | Curved and straight |
| Dressing tissue forceps | SIGMA | F4392 | Length 8 inches |
| Micro-dissecting forceps | SIGMA | F4017 | Straight |
| Micro-dissecting forceps | SIGMA | F4142 | Curved |
| Mayo Scissors | SIGMA | Z265993 | |
| Scalpel | SAKIRA MEDICAL | ||
| Sterile Biopsy Punch Ø 3mm | Stiefel Laboratories Ltd. | 2079D | 5 mm diameter can also be used |
| Sterile 1.5 ml Tubes | Deltalab | 200400P | |
| Sterile 15 ml Tubes | Greiner | 188271 | |
| Sterile 50 ml Tubes | Greiner | 227261 | |
| Sterile serological pipettes 5 ml | Greiner | 606160 | |
| Sterile serological pipettes 10 ml | Greiner | 607160 | |
| Sterile serological pipettes 25 ml | Greiner | 760180 | |
| Biological safety cabinet, class II | Thermo Scientific | 1300 series, type A2 | |
| Micro-Isolator Rack | RAIR IsoSystem | 76144W | Super Mouse 1800 AllerZone |
| Refrigerated Microcentrifuge | Eppendorf | Legend Micro 21R | |
| Microcentrifuge | Heraeus | Biofuge-pico | |
| Centrifuge | Thermo Scientific | Sorval ST40R | |
| CO2 Incubator | Thermo Scientific | Model 3111 | |
| Sterile Thin-tip pasteur pipettes | Deltalab | D210022 | |
| Sterile pasteur pipettes | Deltalab | 200007 | |
| Sterile 24-well plate | Greiner | 662160 | |
| Trypan Blue Solution | Life Technologies | T10282 | |
| Automatic Cell Counter - Countess | Life Technologies | C10227 | |
| Countess Cell Counting Chamber Slides | Life Technologies | C10312 | |
| Flow Cytometry Tubes | BD | 343675 | |
| Flow Cytometer - FACS Canto-II | BD | ||
| Real Time PCR Instrument - Rotor Gene Q or ABI 7900 | Qiagen / Applied Biosystems | ||
| Trizol Reagent | Life Technologies | 15596-026 | Molecular Biology Grade |
| DNAse-I | Life Technologies | 18068-015 | Molecular Biology Grade |
| DNAse-I Buffer 10X | Life Technologies | 18068015 | Molecular Biology Grade |
| EDTA 25 mM | Life Technologies | 18068015 | Molecular Biology Grade |
| Ultra-Pure Water | Life Technologies | 10977 | Molecular Biology Grade |
| RNAse Out | Life Technologies | 100000840 | Molecular Biology Grade |
| Random Hexamer Primers | Life Technologies | N8080127 | Molecular Biology Grade |
| M-MLV-RT buffer | Life Technologies | 18057-018 | Molecular Biology Grade |
| M-MLV-RT enzime | Life Technologies | 28025-021 | Molecular Biology Grade |
| QuantiTect Syber Green PCR Kit | Qiagen | 204143 | Molecular Biology Grade |
| Specific primers | Life Technologies | Molecular Biology Grade |
参考文献
- . Pneumococcal vaccines WHO position paper--2012. Weekly Epidemiological Record. 14, 129-144 (2012).
- Appelbaum, P. C., et al. Carriage of antibiotic-resistant Streptococcus pneumoniae by children in eastern and central Europe-a multicenter study with use of standardized methods. Clin Infect Dis. 23, 712-717 (1996).
- Ramirez, J. A., Anzueto, A. R. Changing needs of community-acquired pneumonia. J Antimicrob Chemother. 66, 3-9 (2011).
- Cuburu, N., et al. Sublingual immunization induces broad-based systemic and mucosal immune responses in mice. Vaccine. 25, 8598-8610 (2007).
- Pedersen, G. K., et al. Evaluation of the sublingual route for administration of influenza H5N1 virosomes in combination with the bacterial second messenger c-di-GMP. PLoS One. 25, 1-12 (2011).
- Song, J. H., et al. Sublingual vaccination with influenza virus protects mice against lethal viral infection. Proc Natl Acad Sci USA. 105, 1644-1649 (2008).
- Cogo, R., Ramponi, A., Scivoletto, G., Rippoli, R. Prophylaxis for acute exacerbations of chronic bronchitis using an antibacterial sublingual vaccine obtained through mechanical lysis: a clinical and pharmacoeconomic study. Acta Biomed. 74, 76-87 (2003).
- Rosaschino, F., Cattaneo, L. Strategies for optimizing compliance of paediatric patients for seasonal antibacterial vaccination with sublingually administered Polyvalent Mechanical Bacterial Lysates (PMBL). Acta Biomed. 75, 171-178 (2004).
- Senna, G., Caminati, M., Canonica, G. W. Safety and tolerability of sublingual immunotherapy in clinical trials and real life. Curr Opin Allergy Clin Immunol. 13, 656-662 (2013).
- Mascarell, L., et al. Oral dendritic cells mediate antigen-specific tolerance by stimulating TH1 and regulatory CD4+ T cells. J Allergy Clin Immunol. 122, 603-609 (2008).
- Mascarell, L., et al. Mapping of the lingual immune system reveals the presence of both regulatory and effector CD4+ T cells. Clin Exp Allergy. 39, 1910-1919 (2009).
- Mascarell, L., et al. Oral macrophage-like cells play a key role in tolerance induction following sublingual immunotherapy of asthmatic mice. Mucosal Immunology. 4, 638-647 (2011).
- Hervouet, C., et al. Antigen-bearing dendritic cells from the sublingual mucosa recirculate to distant systemic lymphoid organs to prime mucosal CD8 T cells. Mucosal Immunology. 7, 280-291 (2014).
- Munoz, N., et al. Mucosal administration of flagellin protects mice from Streptococcus pneumoniae lung infection. Infect Immun. 78, 4226-4233 (2010).
- Hayashi, F., et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 410, 1099-1103 (2001).
- Lightfield, K. L., et al. Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nature Immunology. 9, 1171-1178 (2008).
- Lightfield, K. L., et al. Differential requirements for NAIP5 in activation of the NLRC4 inflammasome. Infect Immun. 79, 1606-1614 (2011).
- Honko, A. N., Mizel, S. B. Mucosal administration of flagellin induces innate immunity in the mouse lung. Infect Immun. 72, 6676-6679 (2004).
- Janot, L., et al. Radioresistant cells expressing TLR5 control the respiratory epithelium's innate immune responses to flagellin. Eur J Immunol. 39 (6), 1587-1596 (2009).
- Van Maele, L., et al. TLR5 signaling stimulates the innate production of IL-17 and IL-22 by CD3(neg)CD127+ immune cells in spleen and mucosa. J Immunol. 185, 1177-1185 (2010).
- Lee, S. J., et al. Neurologic adverse events following influenza A (H1N1) vaccinations in children. Pediatrics international: official journal of the Japan Pediatric Society. 54, 325-330 (2012).
- Lewis, D. J., et al. Transient facial nerve paralysis (Bell's palsy) following intranasal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin. PLoS One. 4, e6999 (2009).
- Mutsch, M., et al. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N Engl J Med. 350, 896-903 (2004).
- Kuo, C. H., Wang, W. L., Chu, Y. T., Lee, M. S., Hung, C. H. Sublingual immunotherapy in children: an updated review. Pediatr Neonatol. 50, 44-49 (2009).
- Nempont, C., Cavet, D., Rumbo, M., Bompard, C., Villeret, V., Sirard, J. C. Deletion of flagellin's hypervariable region abrogates antibody-mediated neutralization and systemic activation of TLR5-dependent immunity. J. Immunol. 181, 2036-2043 (2008).
- Marques, J. M., et al. Protection against Streptococcus pneumoniae serotype 1 acute infection shows a signature of Th17- and IFN-gamma-mediated immunity. Immunobiology. 217, 420-429 (2012).
- Stewart, C. C., Stewart, S. J., et al. Titering antibodies. Current Protocols in Cytometry. 4, Unit 4.1 (2001).
- Kubista, M., et al. The real-time polymerase chain reaction. Molecular Aspects of Medicine. 27, 95-125 (2006).
- Pedersen, G., Cox, R. The mucosal vaccine quandary: intranasal vs. sublingual immunization against influenza. Human Vaccines & Immunotherapeutics. 8, 689-693 (2012).
- Vitaliti, G., et al. Mucosal immunity and sublingual immunotherapy in respiratory disorders. Journal of Biological Regulators and Homeostatic Agents. 26, S85-S93 (2012).
转载和许可
请求许可使用此 JoVE 文章的文本或图形
请求许可探索更多文章
This article has been published
Video Coming Soon
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。