需要订阅 JoVE 才能查看此. 登录或开始免费试用。
Method Article
生产合成核熔化玻璃
摘要
A protocol for the production of synthetic nuclear melt glass, similar to trinitite, is presented.
摘要
Realistic surrogate nuclear debris is needed within the nuclear forensics community to test and validate post-detonation analysis techniques. Here we outline a novel process for producing bulk surface debris using a high temperature furnace. The material developed in this study is physically and chemically similar to trinitite (the melt glass produced by the first nuclear test). This synthetic nuclear melt glass is assumed to be similar to the vitrified material produced near the epicenter (ground zero) of any surface nuclear detonation in a desert environment. The process outlined here can be applied to produce other types of nuclear melt glass including that likely to be formed in an urban environment. This can be accomplished by simply modifying the precursor matrix to which this production process is applied. The melt glass produced in this study has been analyzed and compared to trinitite, revealing a comparable crystalline morphology, physical structure, void fraction, and chemical composition.
引言
Concerns over the potential malicious use of nuclear weapons by terrorists or rogue nations have highlighted the importance of nuclear forensics analysis for the purpose of attribution.1 Rapid post-detonation analysis techniques are desirable to shorten the attribution timeline as much as possible. The development and validation of such techniques requires realistic nuclear debris samples for testing. Nuclear testing no longer occurs in the United States and nuclear surface debris from the testing era is not readily available (with the exception of trinitite - the melt glass produced by the first nuclear test at the trinity site) and therefore realistic surrogate debris is needed.
The primary goal of the method described here is the production of realistic surrogate nuclear debris similar to trinitite. Synthetic nuclear melt glass samples which are readily available to the academic community can be used to test existing analysis techniques and to develop new methods such as thermo-chromatography for rapid post-detonation analysis.2 With this goal in mind the current study is focused on producing samples which mimic trinitite but do not contain any sensitive weapon design information. The fuel and tamper components within these samples are completely generic and the comparison to trinitite is based on chemistry, morphology, and physical characteristics. The similarities between trinitite and the synthetic nuclear melt glass produced in this study have been previously discussed.3
The purpose of this article is to outline the details of the production process used at the University of Tennessee (UT). This production process was developed with two key parameters in mind: 1) the composition of material incorporated into nuclear melt glass, and 2) the melting temperature of the material. Methods exist for estimating the melting temperature of glass forming networks4 and these techniques have been employed here, along with additional experimentation to determine the optimal processing temperature for the trinitite matrix.5
Alternative methods for surrogate debris production have been published recently. The use of high power lasers has the advantage of creating sufficiently high temperatures to cause elemental fractionation within the target matrix.6 Porous chromatographic substrates have been used to produce small particles similar to fallout particles using condensed phase methods7. The latter method is most useful for producing particulate debris (nuclear fallout) and has been demonstrated with natural metals. The advantages of the method presented here are 1) simplicity, 2) reproducibility, and 3) scalability (sample sizes can range from tiny beads to large chunks of melt glass). Also, this method is expandable both in terms of production output and variety of explosive scenarios covered, and it has already been demonstrated using radioactive materials. A sample has been successfully activated at the High Flux Isotope Reactor (HFIR) at Oak Ridge National Laboratory (ORNL). Natural uranium compounds were added to the matrix prior to melting and fission products were produced in situ by neutron irradiation.
Methods within the glass making industry and those employed for the purpose of radioactive waste immobilization8 have been consulted in the development of the method presented here. The unique effects of radiation in glasses are of inherent interest9 and will constitute an important area of study as this method is further developed.
The method described below is appropriate for any application where a bulk melt glass sample is desired. These samples most closely resemble the material found near the epicenter of a nuclear explosion. Samples of various sizes can be produced, however, methods employing plasma torches or lasers will be more useful for simulating fine particulate debris. Also, commercial HTFs do not reach temperatures high enough to cause elemental fractionation for a wide range of elements. This method should be employed when physical and morphological characteristics are of primary importance.
研究方案
注意:此处所概述的方法,包括使用放射性物质(例如,铀六水合硝酸)和几个腐蚀性物质。适当的防护服和设备应使用(包括实验室工作服,手套,保护眼睛和通风橱)样品制备过程中。另外,用于该工作实验室区域应定期进行放射性污染监测。
注意:需要的化学化合物列于表1这种制剂是通过检查以前开发的报道成分数据为trinitite 10这里报告测定的质量分数由平均质量分数为几个不同的trinitite样品10"丢失"的质量。 (该部分不和为1)存在添加燃料,篡改,和其他成分时允许有一定的灵活性。我们的独立的几个trinitite样本的分析表明,石英是唯一的矿物相尚存在trinitite。5因此,石英是包含在我们的标准Trinitite配方(STF)的唯一矿物。虽然其他矿物粒舍利已经报道了trinitite,11这些往往是例外,而不是规则。在一般情况下,石英是在熔融玻璃中发现的唯一的矿物。10,12同样,石英砂是沥青和混凝土的共同组成,这将是在城市核熔融玻璃的形成很重要的。
-4| 场均Trinitite数据 | 标准Trinitite配方(STF) | ||
| 复合 | 质量分数 | 复合 | 质量分数 |
| 二氧化硅 | 6.42x10 -1 | 二氧化硅 | 6.42x10 -1 |
| 的 Al 2 O 3 | 1.43x10 -1 | 的 Al 2 O 3 | 1.43x10 -1 |
| 氧化钙 | 9.64x10 -2 | 氧化钙 | 9.64x10 -2 |
| 的FeO | 1.97x10 -2 | 1.97x10 -2 | |
| 氧化镁 | 1.15x10 -2 | 氧化镁 | 1.15x10 -2 |
| 娜2 O | 1.25x10 -2 | 娜2 O | 1.25x10 -2 |
| K 2 O | 5.13x10 -2 | KOH | 6.12x10 -2 |
| 的MnO | 5.05x10 -4 | 的MnO | |
| 二氧化钛 | 4.27x10 -3 | 二氧化钛 | 4.27x10 -3 |
| 总 | 9.81x10 -1 | 总 | 9.91x10 -1 |
表1列出的化学化合物。
1.准备了科技型中小企业
注:需要的设备包括微量天平,金属铲,陶瓷研钵,通风橱,乳胶手套,实验室外套,和护目镜。
- 混合的非放射性组件
- 获得至少65克石英砂(SiO 2)的15克的 Al 2 O 3的</子>粉末,10g的CaO粉剂的,2克的FeO粉末,将2克的MgO粉末,将2克的Na 2 O粉末,7克KOH颗粒,1克的MnO粉末和1g 的 TiO 2粉末(在表1中列出的化合物)。
- 使用微量天平和小抹刀精确地测量每种化合物的质量分数,如表1所列 。为了达到最佳效果制备100克非放射性前体基质的在同一时间。
- 使用研钵和杵粉碎(至〜:10-20微米大小的颗粒),并充分混合的化合物中,形成含有64.2克的 SiO 2,14.2克的 Al 2 O 3的,9.64克的CaO,1.97克的均匀粉末混合物的FeO,1.15克氧化镁,1.25克的Na 2 O的6.12克氢氧化钾,0.0505克的MnO和0.427克二氧化钛。
- 搅拌该混合物,用球混合器,下一步采取前不久。
- 用铀六水合硝酸混合STF的(UNH)
- Acqu愤怒至少为1g UNH的。
- 内部通风橱中,粉碎几UNH晶体(用研钵和研杵)以形成1-2微米的颗粒的细粉。
- 添加33.75微克UNH每克非放射性前体基质的(这个比率是合适的用于模拟一个简单的武器的1千吨的收率)。13
- 彻底混合粉末混合物,包括UNH,使用研钵和研杵。熔融步骤前不久完成最终混合。
2.生产1-克熔融玻璃样品
注:需要的设备包括额定在1600℃以上,高纯石墨坩埚,长的不锈钢坩埚钳,耐热手套和保护眼睛的HTF。耐热手套和保护眼睛应该引入或从炉中取出样品时佩戴。因为他们减少从炉强光有色护目镜(或太阳眼镜)是有用的。
- 生产非放射性样品
- 填充厚的陶瓷盘(诸如砂浆)与约100克的纯石英砂,并在室温保持邻近那里的样本将被熔化炉的位置。
- 预热导热油〜1500℃。
- 仔细测量将1.00g非放射性粉末混合物并将粉末在高纯度石墨坩埚中。
- 小心地在加热的HTF的坩埚(使用长对钢坩埚钳)和30分钟熔化混合物。
- 取出样品(再次使用夹钳)和熔融样品倒入充满沙子的砂浆。
- 让玻璃珠,以处理前冷却1-2分钟。
- 抛光珠以除去残余的砂(如有必要)。
- 的放射性样品的
- 重复步骤2.1.1和2.1.2以上。
- 仔细衡量1.00克放射性粉末混合物(包括UNH)和放置powd使用一个单独的刮刀和微量天平,以避免交叉污染ER在高纯度石墨坩埚。
- 重复步骤2.1.4 - 2.1.6以上。
- 监控炉子周围的区域(使用手持式辐射检测器和/或刷卡测定)来检查放射性污染。
3.样品激活
注意:下面的公式推导假设使用武器级(浓)金属铀。 UNH或氧化铀的数量将需要根据元素铀的质量分数和铀-235富集水平缩放。
- 一个熔融玻璃样品铀笛的激活
- 计算所需的使用低于 13(其中,m U表示的铀的质量分数和Y表示的武器收率)的方程样品铀金属的质量分数:
473 / 53473eq1.jpg"/> - 择:使用下面的等式计算的篡改(例如,天然铀,铅,钨)的质量分数:13
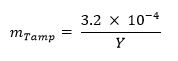
- 计算使用下面的等式13中的样品中的裂变的目标数,其中M s表示在克样品的质量和 N f表示照射期间在样品中产生的裂变的数目:
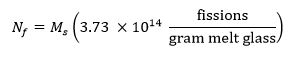
- 用低于 13的方程其中 m 235表示铀-235质量分数(丰度水平)和叔IRR是以秒的照射时间计算所需的照射时间:
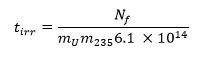
- 照射样品为在4.0×10 14 N / 平方厘米/秒的热中子通量牛逼 IRR秒。例如,在气动管道1(PT-1)在HFIR一个60秒的照射(具有35的热,以共振比)将含有870 UNH微克(相当于410微克的样品中产生约1.1×10 11分体天然铀,或3.0微克的铀-235)。实现这一点的一个0.433克玻璃珠设计成模拟通过用0.1千吨收率武器产生的熔融玻璃样品。该样本已被彻底Cook等14分析。
- 按照适用的安全协议,用于处理放射性样品后照射。
- 计算所需的使用低于 13(其中,m U表示的铀的质量分数和Y表示的武器收率)的方程样品铀金属的质量分数:
- 一个熔融玻璃样品与钚燃料的激活(规划因素)
- 计算钚金属的质量分数所需的使用低于 13,其中m 莆 represen等式样品TS钚质量分数和Y表示的武器收率
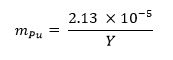
- 重复步骤3.1.2和3.1.3以上。
- 确定,以获得熔融玻璃样品在裂变的期望数量的所需要的照射时间。这个时间将取决于钚的组成和品级以及中子能量光谱。
- 计算钚金属的质量分数所需的使用低于 13,其中m 莆 represen等式样品TS钚质量分数和Y表示的武器收率
注:应十分注意,当钚和其他分析处理将需要采取。在撰写本文时,只有铀已被用于在UT产生和照射HFIR合成熔融玻璃样本。
结果
在这项研究中产生的非放射性样品已相比trinitite和图1-3表明物理性质和形态确实相似。 图1提供了揭示这在宏观水平都观察到颜色和纹理的相似度照片。 图2示出了扫描电子显微镜(SEM)二次电子(SE)的图像其揭示相似的特征在微米级。用SEM和SEM软件进行扫描电镜分析。许多空隙在两个trinitite和合成样品观察。的缺陷和异质在两种以及类似 ,如?...
讨论
注意有关步骤1.2.2和1.2.3:UNH的确切数额将根据场景变化被模拟。通过Giminaro 等人开发规划的公式可以用来选择铀适当的质量作为本文的"示例激活"一节中讨论给定的样本13。此外,氧化铀(UO 2或U 3 O 8)来代替UNH的使用,如果有的话,和铀-235的化合物(UNH或氧化铀是否)必须考虑的质量分数。对于这里所讨论的实验中UNH均匀混合所述前体基质内。可以...
披露声明
This work was performed under grant number DE-NA0001983 from the Stewardship Science Academic Alliances (SSAA) Program of the National Nuclear Security Administration (NNSA).
致谢
Portions of this study have been previously published in the Journal of Radioanalytical and Nuclear Chemistry.3,13 A patent is pending for this method.
材料
| Name | Company | Catalog Number | Comments |
| High Temperature Furnace (HTF) | Carbolite | HTF 18 | 1,800 °C HTF used to melt samples |
| High Temperature Drop Furnace | CM Inc. | 1706 BL | 1,700 °C Drop Furnace used to melt samples |
| Graphite Crucibles | SCP Science | 040-060-041 | 27 ml high purity graphite crucibles (10 pack) |
| Crucible Tongs | Grainger | 5ZPV0 | 26 in., stainless steele tongs for handling crucibles |
| Heat Resistent Gloves | Grainger | 8814-09 | Gloves used to protect hands from heat during sample intro/removal |
| Mortar & Pestle | Fisherbrand | S337631 | 300 ml, Ceramic mortar and pestle for powdering and mixing |
| Micro Balance | Grainger | 8NJG2 | 220 g Cap, high precision scale for measuring powder mass |
| Spatulas | Fisherbrand | 14374 | Metal spatulas for measure small quantities of powder |
| SiO2 | Sigma-Aldrich | 274739-5KG | Quartz Sand CAS Number: 14808-60-7 |
| Al2O3 | Sigma-Aldrich | 11028-1KG | Aluminum Oxide Powder CAS Number: 1344-28-1 |
| CaO | Sigma-Aldrich | 12047-2.5KG | Calcium Oxide Powder CAS Number: 1305-78-8 |
| FeO | Sigma-Aldrich | 400866-25G | Iron Oxide Powder CAS Number: 1345-25-1 |
| MgO | Sigma-Aldrich | 342793-250G | Magnesium Oxide Powder CAS Number: 1309-48-4 |
| Na2O | Sigma-Aldrich | 36712-25G | Sodium Oxide Powder CAS Number: 1313-59-3 |
| KOH | Sigma-Aldrich | 278904-250G | Potasium Hydroxide Pellets CAS Number: 12030-88-5 |
| MnO | Sigma-Aldrich | 377201-500G | Manganese Oxide Powder CAS Number: 1344-43-0 |
| TiO2 | Sigma-Aldrich | 791326-5G | Titanium Oxide Beads CAS Number: 12188-41-9 |
参考文献
- Carnesdale, A. . Nuclear Forensics: A Capability at Risk (Abbreviated Version). , (2010).
- Garrison, J. R., Hanson, D. E., Hall, H. L. Monte Carlo analysis of thermochromatography as a fast separation method for nuclear forensics. J Radioanal Nucl Chem. 291 (3), 885-894 (2011).
- Molgaard, J. J., et al. Development of synthetic nuclear melt glass for forensic analysis. J Radioanal Nucl Chem. 304 (3), 1293-1301 (2015).
- Fluegel, A. Modeling of Glass Liquidus Temperatures using Disconnected Peak Functions. , (2007).
- Oldham, C. J., Molgaard, J. J., Auxier, J. D., Hall, H. L. Comparison of Nuclear Debris Surrogates Using Powder X-Ray Diffraction. , (2014).
- Liezers, M., Fahey, A. J., Carman, A. J., Eiden, G. C. The formation of trinitite-like surrogate nuclear explosion debris ( SNED ) and extreme thermal fractionation of SRM-612 glass induced by high power CW CO 2 laser irradiation. J Radional Nucl Chem. 304 (2), 705-715 (2015).
- Harvey, S. D., et al. Porous chromatographic materials as substrates for preparing synthetic nuclear explosion debris particles. J Radioanal Nucl Chem. 298 (3), 1885-1898 (2013).
- Hanni, J. B., et al. Liquidus temperature measurements for modeling oxide glass systems relevant to nuclear waste vitrification. J Mater Res. 20 (12), 3346-3357 (2005).
- Weber, W. J., et al. Radiation Effects in Glasses Used for Immobilization of High-Level Waste and Plutonium Disposition. J Mater Res. 12 (8), 1946-1978 (1997).
- Eby, N., Hermes, R., Charnley, N., Smoliga, J. A. Trinitite-the atomic rock. Geol Today. 26 (5), 180-185 (2010).
- Bellucci, J. J., Simonetti, A. Nuclear forensics: searching for nuclear device debris in trinitite-hosted inclusions. J Radioanal Nucl Chem. 293 (1), 313-319 (2012).
- Ross, C. S. . Optical Properties of Glass from Alamogordo, New Mexico. , (1948).
- Giminaro, A. V., et al. Compositional planning for development of synthetic urban nuclear melt glass. J Radional Nucl Chem. , (2015).
- Cook, M. T., Auxier, J. D., Giminaro, A. V., Molgaard, J. J., Knowles, J. R., Hall, H. L. A comparison of gamma spectra from trinitite versus irradiated synthetic nuclear melt glass. J Radioanal Nucl Chem. , (2015).
- Fahey, J., Zeissler, C. J., Newbury, D. E., Davis, J., Lindstrom, R. M. Postdetonation nuclear debris for attribution. Proc Natl Acad Sci U S A. 107 (47), 20207-20212 (2010).
- Bellucci, J. J., Simonetti, A., Koeman, E. C., Wallace, C., Burns, P. C. A detailed geochemical investigation of post-nuclear detonation trinitite glass at high spatial resolution: Delineating anthropogenic vs. natural components. Chem Geol. 365, 69-86 (2014).
- Donohue, P. H., Simonetti, A., Koeman, E. C., Mana, S., Peter, C. Nuclear Forensic Applications Involving High Spatial Resolution Analysis of Trinitite Cross-Sections. J Radioanal Nucl Chem. , (2015).
- Eaton, G. F., Smith, D. K. Aged nuclear explosive melt glass: Radiography and scanning electron microscope analyses documenting radionuclide distribution and glass alteration. J Radioanal Nucl Chem. 248 (3), 543-547 (2001).
- Kersting, A. B., Smith, D. K. . Observations of Nuclear Explosive Melt Glass Textures and Surface Areas. , (2006).
- . . IAEA Safeguards Glossary. , (2001).
- Glasstone, S., Dolan, P. . Effects of Nuclear Weapons. , (1977).
- Carney, K. P., Finck, M. R., McGrath, C. A., Martin, L. R., Lewis, R. R. The development of radioactive glass surrogates for fallout debris. J Radioanal Nucl Chem. 299 (1), 363-372 (2013).
- Molgaard, J. J., Auxier, J. D., Hall, H. L. A Comparison of Activation Products in Different Types of Urban Nuclear Melt Glass. , (2015).
转载和许可
请求许可使用此 JoVE 文章的文本或图形
请求许可探索更多文章
This article has been published
Video Coming Soon
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。