收养细胞转移:将供体小鼠孢子细胞引入宿主小鼠,并通过FACS评估成功
Overview
资料来源: 穆尼尔·西尔万1,2,3,佩尔切特·蒂鲍特1,2,3,苏菲·诺沃4,雷切尔·戈卢布1,2,3
1法国巴黎巴斯德研究所免疫学系淋巴病系
2 INSERM U1223, 巴黎, 法国
3巴黎迪德罗大学,索邦巴黎城,大提琴巴斯德,巴黎,法国
4流式细胞测定,细胞学和生物标志物 UtechS,转化科学中心,巴斯德研究所,法国巴黎
采用细胞转移是一种将细胞引入患者或研究生物体以治疗疾病或研究生物过程(如造血细胞)的方法。收养转让的目的多种多样;它可用于基础生物学以及医学(1,2)。在小鼠模型中,可以研究转移细胞的迁移和分布,然后跟踪系统(细胞表面标记、CFSE染色等)。在小鼠模型的癌症研究中,特定细胞群的转移可用作肿瘤的实验治疗。这项技术的另一个例子是通过将骨髓细胞转移到辐照小鼠或患有严重免疫缺陷表型的小鼠,从而产生嵌合小鼠。例如,此小鼠模型可用于评估基因删除对特定细胞群的影响。骨借用细胞的转移也用于人体医疗。当患者在癌症治疗时被照射时,骨髓的接受转移允许免疫系统重组。
此技术的第一步是获得感兴趣的细胞群。选择隔离此人群的技术取决于目标人群的特异性水平。最大的选择水平是整个器官,其中所有细胞群存在于器官。更精确的方法是选择目标细胞群,通常由一个细胞表面标记选择。在这种情况下,对单元格进行排序的理想方法是通过磁性排序。最后,最严格的级别是通过几个细胞表面标记选择细胞来对非常具体的细胞群进行排序。流式细胞分集是这种选择级别最常用的方法。一旦获得感兴趣的人口,就可以将其转移到主机。在收养转移之前,必须确保宿主和捐赠者之间的兼容性。事实上,无论转移目标如何,兼容性对于确保宿主采用细胞而不排斥细胞至关重要。
在本实验练习中,我们演示了采用细胞转移技术,方法是将CD45.2小鼠的细胞转移到CD45.1 Rag_c小鼠(缺乏淋巴细胞),四天后使用流细胞测定确认细胞转移(参见图1).
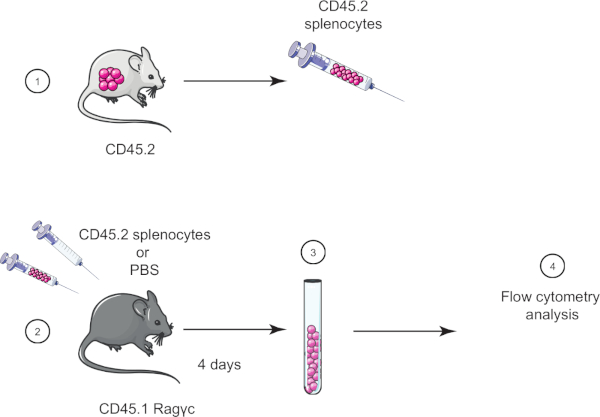
图1:收养转移的原理表。(1)从CD45.2小鼠中分离出孢子细胞,(2)在CD45.1 Rag_c小鼠中转移,仅给对照小鼠注射PBS。(3)收养转移后4天,从小鼠中恢复sssa细胞,(4)通过流动细胞学进行分析。请点击此处查看此图的较大版本。
Procedure
1. 准备
- 开始之前,戴上实验室手套和适当的防护服。
- 消毒所有解剖工具,首先用洗涤剂,然后用70%乙醇,然后彻底干燥。
- 准备 50 mL 的汉克平衡盐溶液 (HBSS) 含有 2% 胎儿小牛血清 (FCS)。
2. 解剖
- 使用二氧化碳输送系统,用缺氧对小鼠实施安乐死。将安乐死小鼠固定在上部位置的解剖板上,并使用剪刀和钳子进行纵向腹腔切除术。
- 使用钳子移动腹部右侧的肠道和胃,露出胃和脾脏。脾脏附着在胃上。
- 使用钳子小心地从胃分离脾脏,并将其放在含有 5 mL 的 HBSS 2% FCS 的培养皿上。
3. 免疫细胞隔离
- 将脾脏放在培养皿上的 40 μm 细胞滤网上。用柱塞压碎脾脏以将其分离。
- 用 1 mL 的 HBSS 2% FCS 对柱塞和滤网进
Results
Application and Summary
References
- Restifo, N. P., Dudley, M. E. and Rosenberg., S. A. Adoptive immunotherapy for cancer: harnessing the T cell response. Nature reviews. Immunology, 12 (4): 269-281, (2012).
- Bonini, C., and Mondino, A. Adoptive T-cell therapy for cancer: The era of engineered T cells. European journal of immunology, 45 (9): 2457-69, (2015).
Tags
跳至...
此集合中的视频:

Now Playing
收养细胞转移:将供体小鼠孢子细胞引入宿主小鼠,并通过FACS评估成功
Immunology
22.3K Views

流细胞学和荧光活化细胞分拣(FACS):血性B淋巴细胞的分离
Immunology
92.9K Views

磁性活细胞分拣 (MACS): 胸腺 T 淋巴细胞的分离
Immunology
22.9K Views

ELISA Asas :间接、三明治和竞争
Immunology
238.3K Views

ELISPOT 分析:检测 IFN-- 分泌性细胞
Immunology
28.5K Views

免疫组织化学和免疫细胞化学:通过光显微镜进行组织成像
Immunology
78.9K Views

抗体生成:使用杂交瘤生产单克隆抗体
Immunology
43.5K Views

免疫荧光显微镜:石蜡内嵌组织部分的免疫荧光染色
Immunology
53.8K Views

共聚焦荧光显微镜:确定小鼠纤维细胞中蛋白质定位的技术
Immunology
43.2K Views

基于免疫沉淀的技术:使用阿加玫瑰珠纯化内源性蛋白质
Immunology
87.7K Views

细胞周期分析:使用CFSE染色和流细胞测定法评估刺激后CD4和CD8 T细胞增殖
Immunology
24.2K Views

细胞死亡测定:细胞毒性能力的铬释放测定
Immunology
151.4K Views
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。