Hess’s law can be used to determine the enthalpy change of any reaction if the corresponding enthalpies of formation of the reactants and products are available. The main reaction may be divided into stepwise reactions : (i) decompositions of the reactants into their component elements, for which the enthalpy changes are proportional to the negative of the enthalpies of formation of the reactants, −ΔHf°(reactants), followed by (ii) re-combinations of the elements (obtained in step 1) to give the products, with the enthalpy changes proportional to the enthalpies of formation of the products, ΔHf° (products). The standard enthalpy change of the overall reaction is therefore equal to: (ii) the sum of the standard enthalpies of formation of all the products plus (i) the sum of the negatives of the standard enthalpies of formation of the reactants, as given by the following equation, where ∑ represents “the sum of” and n stands for the stoichiometric coefficients.
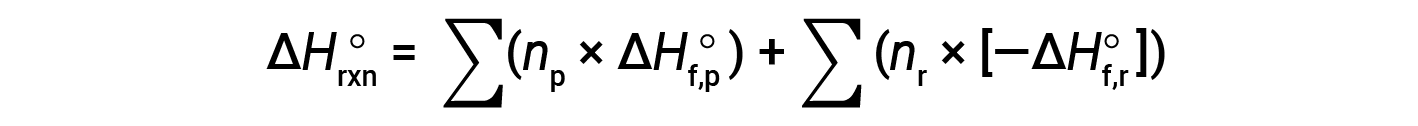
The equation is usually rearranged slightly to be written as follows:
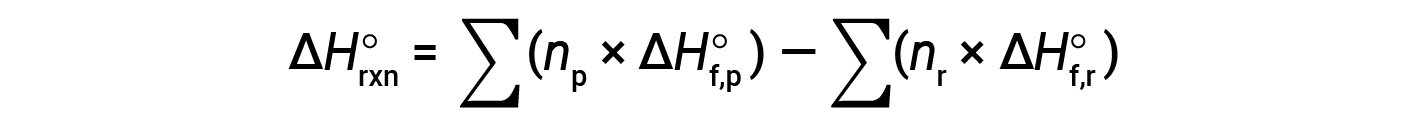
The following example shows in detail why this equation is valid and how to use it to calculate the standard enthalpy change for a reaction:

Here, the special form of Hess’s law and the heat of formation values for the reactants and products is used: ΔHf° (HNO3) = −206.64 kJ/mol; ΔHf° (NO) = +90.2 kJ/mol; ΔHf° (NO2) = +33 kJ/mol; ΔHf° (H2O) = −285.8 kJ/mol.


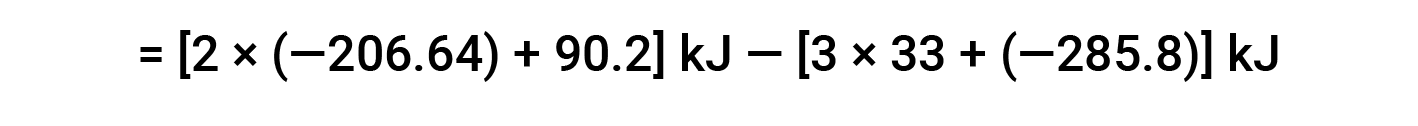
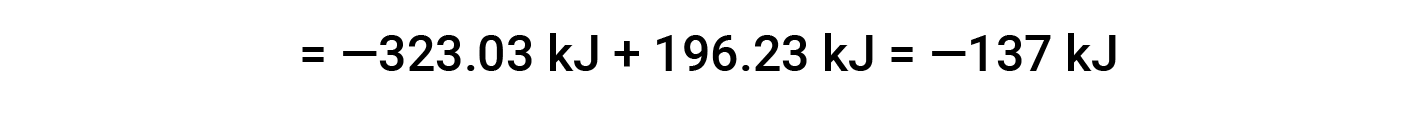
This text is adapted from Openstax, Chemistry 2e, Section 5.3: Enthalpy.
Aus Kapitel 6:

Now Playing
6.12 : Reaktionsenthalpie
Thermochemie
31.2K Ansichten

6.1 : Energiegrundlagen
Thermochemie
36.2K Ansichten

6.2 : Erster Hauptsatz der Thermodynamik
Thermochemie
30.3K Ansichten

6.3 : Innere Energie
Thermochemie
28.2K Ansichten

6.4 : Quantifizierung von Wärme
Thermochemie
52.7K Ansichten

6.5 : Quantifizierung von Arbeit
Thermochemie
18.7K Ansichten

6.6 : Enthalpie
Thermochemie
34.2K Ansichten

6.7 : Thermochemische Gleichungen
Thermochemie
27.8K Ansichten

6.8 : Konstantdruck-Kalorimetrie
Thermochemie
83.3K Ansichten

6.9 : Kalorimetrie bei konstantem Volumen
Thermochemie
26.6K Ansichten

6.10 : Hessscher Wärmesatz
Thermochemie
43.6K Ansichten

6.11 : Standardbildungsenthalpie
Thermochemie
40.4K Ansichten
Copyright © 2025 MyJoVE Corporation. Alle Rechte vorbehalten