A system at equilibrium is in a state of dynamic balance, with forward and reverse reactions taking place at equal rates. If an equilibrium system is subjected to a change in conditions that affects these reaction rates differently (a stress), then the rates are no longer equal and the system is not at equilibrium. The system will subsequently experience a net reaction in the direction of a greater rate (a shift) that will re-establish the equilibrium. This phenomenon is summarized by Le Châtelier’s principle: if an equilibrium system is stressed, the system will experience a shift in response to the stress that re-establishes equilibrium.
Reaction rates are affected primarily by concentrations, as described by the reaction’s rate law, and temperature, as described by the Arrhenius equation. Consequently, changes in concentration and temperature are the two stresses that can shift an equilibrium.
Effect of a Change in Concentration
If an equilibrium system is subjected to a change in the concentration of a reactant or product species, the rate of either the forward or the reverse reaction will change. As an example, consider the equilibrium reaction:

When this system is at equilibrium, the forward and reverse reaction rates are equal.
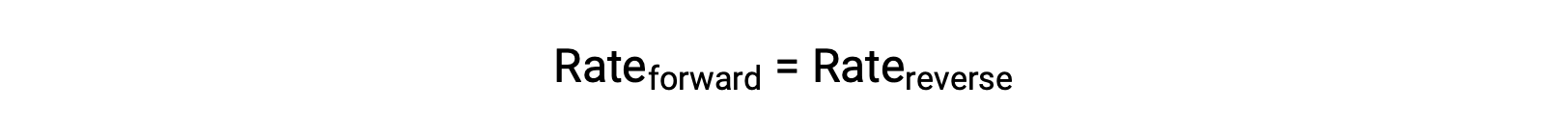
If the system is stressed by adding reactant, either N2 or O2, the resulting increase in concentration causes the rate of the forward reaction to increase, exceeding that of the reverse reaction:

The system will experience a temporary net reaction in the forward direction to re-establish equilibrium (the equilibrium will shift right). This same shift will result if some product NO is removed from the system, which decreases the rate of the reverse reaction, again resulting in the same imbalance in rates.
The same logic can be used to explain the left shift that results from either removing reactant or adding product to an equilibrium system. Both of these stresses result in an increased rate for the reverse reaction

and a temporary net reaction in the reverse direction to re-establish equilibrium.
As an alternative to this kinetic interpretation, the effect of changes in concentration on equilibria can be rationalized in terms of reaction quotients. When the system is at equilibrium,
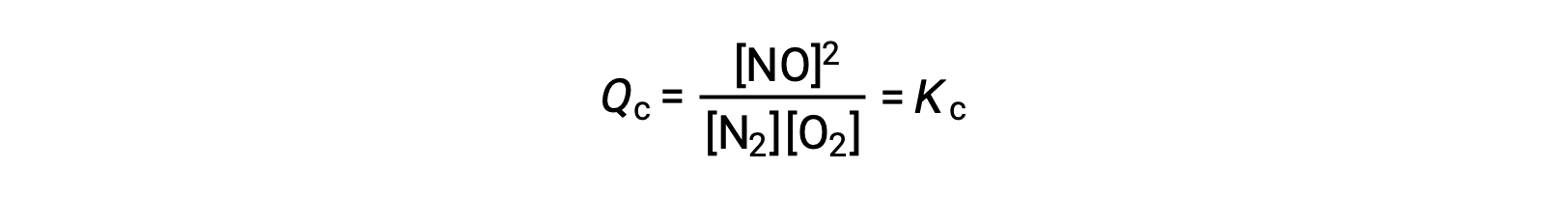
If a reactant is added (increasing the denominator of the reaction quotient) or product is removed (decreasing the numerator), then Qc < Kc and the equilibrium will shift right. Note that the three different ways of inducing this stress result in three different changes in the composition of the equilibrium mixture. If N2 is added, the right shift will consume O2 and produce NO as equilibrium is re-established, yielding a mixture with a greater concentration of N2 and NO and a lesser concentration of O2 than was present before. If O2 is added, the new equilibrium mixture will have greater concentrations of O2 and NO and a lesser concentration of N2. Finally, if NO is removed, the new equilibrium mixture will have greater concentrations of N2 and O2 and a lesser concentration of NO. Despite these differences in composition, the value of the equilibrium constant will be the same after the stress as it was before (per the law of mass action). The same logic may be applied for stresses involving removing reactants or adding product, in which case Qc > Kc and the equilibrium will shift left.
This text has been adapted from Openstax, Chemistry 2e, Section 13.3 Shifting Equilibria: LeChatelier’s Principle.
Tags
Aus Kapitel 14:

Now Playing
14.7 : Le Chatelier's Principle: Changing Concentration
Chemisches Gleichgewicht
56.4K Ansichten

14.1 : Fließgleichgewicht
Chemisches Gleichgewicht
48.5K Ansichten

14.2 : Die Gleichgewichtskonstante
Chemisches Gleichgewicht
45.0K Ansichten

14.3 : Homogene Gleichgewichte für gasförmige Reaktionen
Chemisches Gleichgewicht
23.3K Ansichten

14.4 : Berechnung der Gleichgewichtskonstante
Chemisches Gleichgewicht
29.8K Ansichten

14.5 : Reaktionsquotient
Chemisches Gleichgewicht
47.1K Ansichten

14.6 : Berechnung von Gleichgewichtskonzentrationen
Chemisches Gleichgewicht
46.1K Ansichten

14.8 : Das Prinzip von Le Chatelier: Ändern des Volumens (Druck)
Chemisches Gleichgewicht
33.2K Ansichten

14.9 : Das Prinzip von Le Chatelier: Temperaturwechsel
Chemisches Gleichgewicht
28.2K Ansichten

14.10 : Die kleine x Annahme
Chemisches Gleichgewicht
45.3K Ansichten
Copyright © 2025 MyJoVE Corporation. Alle Rechte vorbehalten