Iniciar sesión
Se requiere una suscripción a JoVE para ver este contenido. Inicie sesión o comience su prueba gratuita.
Methods Article
Preparación de un hexaheliceno funcionalizado-Corannulene por cobre (I) catalizada cicloadición Alquino-azida de Unidades no planas poliaromáticos
En este artículo
Resumen
A continuación, se presenta un protocolo para sintetizar un compuesto orgánico complejo compuesto por tres unidades poliaromáticos no planas, montado fácilmente con rendimientos razonables.
Resumen
The main purpose of this video is to show 6 reaction steps of a convergent synthesis and prepare a complex molecule containing up to three nonplanar polyaromatic units, which are two corannulene moieties and a racemic hexahelicene linking them. The compound described in this work is a good host for fullerenes. Several common organic reactions, such as free-radical reactions, C-C coupling or click chemistry, are employed demonstrating the versatility of functionalization that this compound can accept. All of these reactions work for planar aromatic molecules. With subtle modifications, it is possible to achieve similar results for nonplanar polyaromatic compounds.
Introducción
Debido a su geometría especial, corannulene y helicenes son moléculas que pueden adoptar una estructura lejos de planaridad y dan lugar a propiedades interesantes. 1-15 En los últimos años, la búsqueda de los receptores moleculares de los nanotubos de carbono y fulerenos es un área muy activa 16-19, debido, principalmente, a sus potenciales aplicaciones como materiales para células solares orgánicas, transistores, sensores y otros dispositivos. 20-28 la excelente complementariedad de forma entre corannulene y un fullereno han atraído la atención de varios investigadores con el objetivo de diseñar receptores moleculares capaces de establecer la asociación supramolecular por las fuerzas de dispersión. 29-39
La química de los compuestos poliaromáticos no planas mencionadas anteriormente es similar al descrito para las moléculas totalmente planas, pero a veces es difícil encontrar condiciones adecuadas para alcanzar selectividades y rendimientos deseados. 40 En este trabajo se presenta la síntesis de una molécula (7) que tiene tres unidades poliaromáticos en algunos pasos con buenos rendimientos mediante la aplicación de técnicas sencillas y típicas que se encuentran en todos los laboratorios de investigación. La molécula es de gran importancia, ya que puede adoptar una conformación de pinza para establecer buenas interacciones con C 60 37 en la solución; y puede abrir una línea de investigación como un receptor potencial para altos fullerenos quirales gracias al enlazador heliceno, que es una molécula quiral debido a la existencia de un eje estereogénico. 41-45 Sin embargo, sólo heliceno racémica se utilizará en este trabajo.
En este punto, la única limitación para sintetizar estos receptores es la preparación de helicenes y corannulenes, ya que no están disponibles comercialmente. Pero, de acuerdo con los nuevos métodos publicados en otro lugar 46 a 48 se pueden obtener en cantidades adecuadas en un corto período de tiempo razonable.
Protocolo
1. Funcionalización de 2,15-Dimethylhexahelicene
- Dibromación de 2,15-dimethylhexahelicene
- Pesar 0,356 g (1,0 mmol) de 2,15-dimethylhexahelicene, 0,374 g (2,1 mmol) de bromosuccinimida recién recristalizado N (NBS) y 24 mg (0,07 mmol) de peróxido de benzoilo (BPO) (70% en peso con 30% de agua como estabilizador). Coloque todos los sólidos en un matraz de Schlenk de 100 ml con una barra de agitación magnética. Puesto bajo atmósfera de nitrógeno mediante tres ciclos de evacuación de gases, seguido por el rellenado de gas inerte en la línea de Schlenk.
- Añadir 21 ml de tetracloruro de carbono (CCl 4). Desgasificar la solución por el mismo evacuación / proceso de recarga (etapa 1.1.1) con agitación vigorosa y con cuidado a fin de evitar la pérdida masiva de disolvente.
- Se calienta a reflujo (77 ° C) la mezcla con un baño de aceite durante 4 horas. Comprobar la reacción mediante resonancia magnética nuclear de 1H (RMN). Dobletes entre 3,7 ppm y 4,0 ppm shoparece ULD. Ellos indican la presencia de -CH diastereotópicos 2 - grupos (Figura 1).
- Una vez terminado, se enfría la mezcla a temperatura ambiente y eliminar el disolvente bajo vacío. Configurar una trampa llena de nitrógeno líquido para evitar la contaminación de la bomba.
- Se vuelve a disolver el crudo en 30 ml de diclorometano (DCM), transferir a un matraz de fondo redondo y se mezcla con 4 g de gel de sílice (típicamente agregar 5 veces el peso bruto). Se concentra la mezcla en un evaporador rotatorio.
- Mientras tanto, llenar una columna (longitud de alrededor de 20 cm y un espesor de 4,5 cm) con SiO 2 gel mezclado previamente con hexano / acetato de etilo (95: 5) como fase móvil. Añadir la mezcla a la parte superior de la columna y a continuación, añadir una capa de arena (2 cm).
- Con cuidado, vierta en la nueva fase móvil y llevar a cabo la cromatografía mediante la recopilación de las fracciones en tubos de ensayo (típicamente 20 ml por tubo y cerca de 4 ml de la elución producto esperado). Compruebe fracciones por cromatografía en capa fina(TLC) con la misma fase móvil (hexano / acetato de etilo 95: 5) y la imagen bajo luz UV. El producto esperado (4b) debe eluir con un factor de retención (Rf) de 0,35 como un aceite amarillo después de combinar todos querían fracciones y la eliminación del disolvente en el evaporador rotativo. se deben obtener 334 mg (rendimiento 65%).
NOTA: Todas las técnicas de Schlenk, el uso de un baño de aceite para la configuración de cromatografía de calefacción y de columna será ampliamente utilizados en la mayoría de los protocolos, por lo que de ahora en adelante, no se trata en detalle y sólo unos pocos comentarios, cuando sea necesario, ser dado.
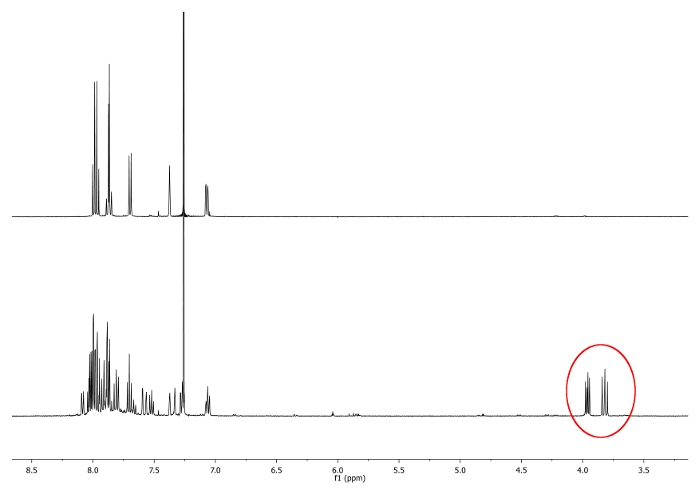
. Figura 1 1 H-NMR espectros (500 MHz, CDCl3) de 2,15 -dimethylhelicene (arriba) y una alícuota tomada después de 2 horas Nuevas señales, que corresponde a -CH 2 -.., se representan en un círculo rojo (inferior) Haga clic aquí para ver una versión más grande de esta figura.
- Síntesis de 2,15-bis (azidometil) hexaheliceno
- Pesar 0,103 g (0,2 mmol) de 2,15-bis (bromometil) hexaheliceno y 0,390 g (6 mmol) de azida de sodio. Coloque ambos sólidos en un matraz Schlenk de 50 ml equipado con una barra magnética y poner bajo una atmósfera de nitrógeno.
- Mezclar 8,6 ml de tetrahidrofurano (THF) con 5,2 ml de agua (H 2 O) y verter la mezcla de disolventes en el matraz de Schlenk. Desgasificar la solución.
- Se calienta a reflujo (65 ° C) durante 3 horas. Compruebe la reacción por 1H-RMN. CH2 - señales deben desplazar a 3,75 ppm (Figura 2).
- Posteriormente, cool por la mezcla a temperatura ambiente y eliminar el THF bajo vacío. Se diluye con 50 ml de H 2 O.
- Transferir la mezcla a un embudo de separación y se extrajo tres veces con 40 ml de DCM. Combinar todas las fases orgánicas y se lava con pura H2O (50 ml).
- Se purificó el crudo por cromatografía en columna sobre gel de sílice utilizando hexano / acetato de etilo (85:15) como fase móvil para dar un aceite de color amarillo a Rf = 0,38 que corresponde a hexaheliceno 2,15-bis (azomethyl) (5b). se deben obtener 70 mg (rendimiento 80%).
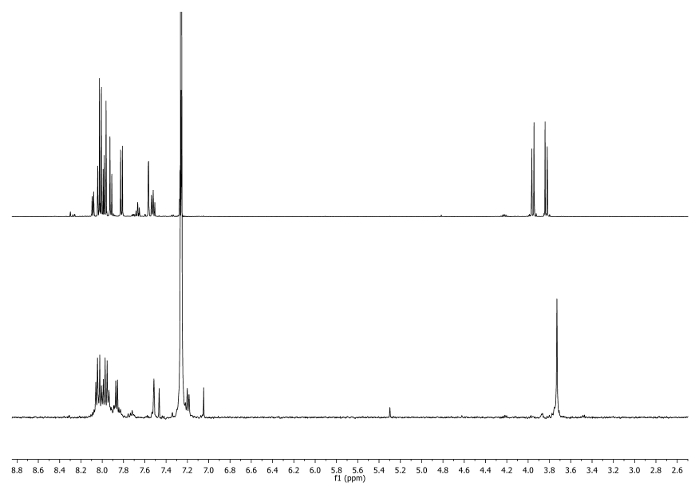
Figura 2: 1H-NMR espectros (500 MHz, CDCl3) del 4 b (parte superior) y una alícuota tomada después de 3 horas(parte inferior). Tenga en cuenta los cambios en la región alifática. Haga clic aquí para ver una versión más grande de esta figura.
2. Funcionalización de Corannulene
- Monobromación de Corannulene
- Pesar 0,125 g (0,5 mmol) de corannulene, 89 mg (0,5 mmol) de NBS recién recristalizado y 17 mg de oro (III) hidrato de cloruro.
- Coloque todos los compuestos en un vial de 10 ml diseñados especialmente para las reacciones de microondas provisto de una barra magnética y luego se ponen en una atmósfera de nitrógeno con la ayuda de un matraz de fondo redondo de 2 cuellos.
- Añadir 7 ml de 1,2-dicloroetano (DCE) y desgasificar la solución.
- Sonicar la mezcla durante 2 min para dispersar partículas de sal de oro.
- El calor en el interior del reactor de microondas a 100 ° C durante 2 hr.
- Cuando haya terminado, transferir el crudo a un matraz de fondo redondo y eliminar el disolvente por rotary evaporación.
- Se purificó el crudo por cromatografía en columna sobre SiO2 gel usando hexano como fase móvil.
NOTA: Bromocorannulene (4a) se obtiene como un sólido de color amarillo a Rf = 0,38. se deben obtener 99 mg (rendimiento 60%). Corannulene sin reaccionar (3a) se puede recuperar y se almacena para usos adicionales. Parece a Rf = 0,29.
- El acoplamiento de Sonogashira Bomocorannulene y etiniltrimetilsilano
- Pesar 49 mg (0,15 mmol) de bromocorannulene, 11 mg (0,015 mmol) de [PdCl 2 (dppf)] 49,50 (dppf ser 1,1-'bis (diphenylphsphino) ferroceno, 3 mg (0,015 mmol) de CuI. 51
- Coloque todos los sólidos en un matraz de Schlenk de 50 ml junto con una barra magnética y poner bajo una atmósfera de nitrógeno.
- Añadir 5,0 ml de trietilamina (NEt 3) y desgasificar la mezcla.
- Por último, añadir 104 l (0,75 mmol) de etiniltrimetilsilano.
- Sonicar la mezcla durante 2 min adispersar partículas de sal de metal.
- Se calienta a 85 ° C durante 24 hr con sonicación periódica para prevenir la deposición de sales metálicas.
NOTA: El color de la mezcla se volvió a negro pronto, lo que indica la presencia de paladio (0). - Enfriar a temperatura ambiente y se evapora NEt 3 al vacío.
- Se vuelve a disolver en 20 ml de DCM y se purifica por cromatografía en columna sobre gel de sílice eluyendo con hexano para dar un sólido de color amarillo a Rf = 0,28 que corresponde a 5a. se deben obtener 41 mg (rendimiento 78%).
NOTA: Si el crudo se filtra a través de una almohadilla de Celite en DCM, se podría obtener una muestra pura razonable, sin embargo los derivados de fosfina no se eliminan totalmente.
- Preparación de Ethynylcorannulene por desprotección TMS
- Pesar 35 mg (0,10 mmol) de 5a y 7,3 mg (0,125 mmol) de fluoruro de potasio anhidro.
- Colocar todos los sólidos en un 50 ml matraz Schlenk equipado con una barra magnética de unad poner bajo atmósfera de nitrógeno.
- Mezclar 4 ml de THF y 4 ml de metanol (MeOH) y se vierte la mezcla en el matraz de Schlenk. Degas a fondo.
- Dejar en reposo a temperatura ambiente, Mantener el matraz protegido de la luz cubriéndola con una película opaca. Compruebe la reacción por 1H-RMN examinado 3,48 ppm. Una señal que corresponde a -CCH debe surgir (Figura 3).
NOTA: A pesar de que este compuesto tiene un alquino terminal que es reactivo y se descompone fácilmente, no se encontraron problemas durante el trabajo arriba descrito a continuación. Se llevó a cabo bajo la luz natural. - Una vez terminado, retirar el THF al vacío y se diluye con 10 ml de agua, transferir todo a un embudo de separación.
- Se extrae con DCM (3 x 15 ml), se combinan todas las fases orgánicas en un matraz de fondo redondo y se concentra en un evaporador rotatorio a temperatura ambiente para obtener finalmente un sólido amarillo correspondiente a 6a. 27 mg deben obtenerse (rendimiento cuantitativo).
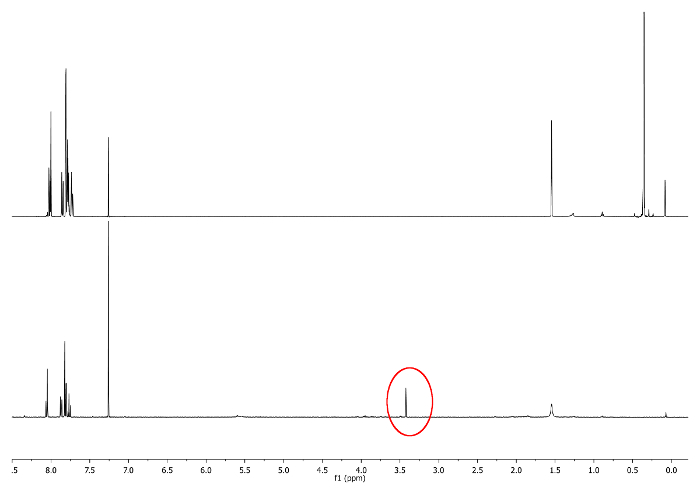
Figura 3:.. 1 H-NMR espectros (500 MHz, CDCl3) del 5 al (arriba) y 6 bis (parte inferior) singlete -CCH se representa en un círculo rojo Haga clic aquí para ver una versión más grande de esta figura.
3. Montaje Final por Click Química
- Pesar 15,3 mg (0,035 mmol) de 5B, 20,0 mg (0,073 mmol) de 6a, 1,4 mg (0,007 mmol) de sal sódica de ácido ascórbico, 1,7 mg (0,007 mmol) de CuSO 4 · 5H 2 O.
- Coloque todos los sólidos en un matraz Schlenk de 50 ml equipado con amagnetic barra y poner bajo una atmósfera de nitrógeno.
- Mezclar 3 ml de H2O y 12 ml de THF y se vierte la mezcla en el matraz de Schlenk. Desgasificar la solución a fondo.
- Se calienta a 65 ° C durante 3 días con un condensador conectado a la parte superior del matraz y comprobar periódicamente la reacción para controlar la temperatura, la agitación y el volumen de disolvente. Compruebe la reacción por 1H-RMN. La señal en 3,48 ppm debe desaparecer y ser desplazado a 7,27 ppm que indica el consumo de corannulene etinilo y la existencia de la unidad de triazol (Figura 4).
- Cuando haya terminado, eliminar el THF bajo vacío y se diluye con 20 ml de agua, la transferencia de la mezcla a un embudo de separación.
- Se extrae con DCM (3 x 20 ml), se combinan todas las fases orgánicas en un matraz de fondo redondo y se concentra en un evaporador rotatorio.
- Se purificó el crudo por cromatografía en columna sobre SiO2 gel eluyendo con hexano / acetato de etilo (1: 1) para dar un sólido de color amarillo pálido a Rf = 0,59correspondiente a 7. se deben obtener 27 mg (rendimiento 75%).
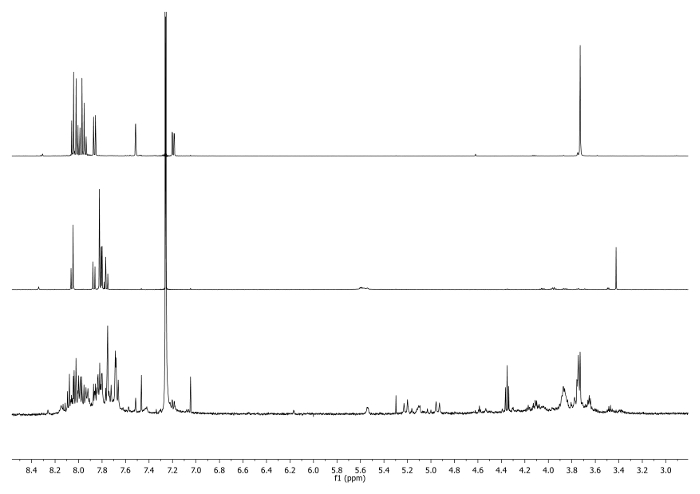
Figura 4: 1H-NMR espectros (500 MHz, CDCl3) del 5 b (parte superior), 6 bis (medio) y una alícuota tomada después de 2 días (abajo) Tenga en cuenta la desaparición de la señal -CCH en el crudo.. Por favor, clic aquí para ver una versión más grande de esta figura.
Resultados
Corannulene (3 a) y 2,15-dimethylhexahelicene (3 b) se pueden preparar siguiendo los métodos actuales 46-48 de una manera sencilla con muy buenos rendimientos (Figura 5). Ambos comparten una molécula común, 2,7-dimetilnaftaleno, como material de partida, dando lugar a una divergente a la síntesis convergente de la molécula final.
Discusión
El compuesto final 7 se ha preparado después de 6 pasos de precursores aromáticos policíclicos no planas 3 y 3b con moderada a muy buenos rendimientos en cada reacción. La principal limitación observada en esta ruta fue la bromación de ambos compuestos poliaromáticos no planas. Sin embargo, en el caso del compuesto 4 a, una cantidad importante de corannulene libre se puede recuperar para otros us...
Divulgaciones
The authors have nothing to disclose.
Agradecimientos
This work was funded by the Spanish Ministerio de Economìa y Competitividad (CTQ 2013-41067-P). H.B. acknowledge with thanks a MEC-FPI grant.
Materiales
| Name | Company | Catalog Number | Comments |
| 2,15-Dimethylhexahelicene | N/A | N/A | Prepared according to reference 5b,c in the main text. |
| Corannulene | N/A | N/A | Prepared according to reference 5a in the main text. |
| N-Bromosuccinimide (NBS) | Sigma Aldrich | B8.125-5 | ReagentPlus®, 99%. Recrystallized from hot water. |
| Benzoyl peroxide (BPO) | Sigma Aldrich | B-2030 | ~70% (titration). 30% water as stabilizer. |
| Sodium azide | Sigma Aldrich | S2002 | ReagentPlus®, ≥99.5%. |
| Gold (III) chloride Hydrate | Sigma Aldrich | 50778 | puriss. p.a., ACS reagent, ≥49% Au basis. |
| Ethynyltrimethylsilane | Sigma Aldrich | 218170 | 98%. |
| [PdCl2(dppf)] | N/A | N/A | Prepared according to reference 6 in the main text. |
| CuI | N/A | N/A | Prepared according to reference 7 in the main text. |
| KF | Sigma Aldrich | 307599 | 99%, spray-dried. |
| (+)-Sodium L-ascorbate | Fluka | 11140 | BioXtra, ≥99.0% (NT). |
| Copper(II) Sulphate 5-hydrate | Panreac | 131270 | for analysis. |
| Carbon tetrachloride (CCl4) | Fluka | 87030 | for IR spectroscopy, ≥99.9%. |
| Dichloromethane (DCM) | Fisher Scientific | D/1852/25 | Analytical reagent grade. Distilled prior to use. |
| Hexane | Fisher Scientific | H/0355/25 | Analytical reagent grade. Distilled prior to use. |
| Ethyl acetate | Scharlau | AC0145025S | Reagent grade. Distilled prior to use. |
| Tetrahydrofuran (THF) | Fisher Scientific | T/0701/25 | Analytical reagent grade. Distilled prior to use. |
| 1,2-Dichloroethane (DCE) | Sigma Aldrich | D6,156-3 | ReagentPlus®, 99%. |
| Methanol (MeOH) | VWR | 20847.36 | AnalaR NORMAPUR. |
| Triethyl amine (NEt3) | Sigma Aldrich | T0886 | ≥99%. |
| Silica gel | Acros | 360050010 | Particle size 40-60mm. |
| Sand - low iron | Fisher Scientific | S/0360/63 | General purpose grade. |
| TLC Silica gel 60 F254 | Merck | 1.05554.0001 | |
| Monowave 300 (Microwave reactor) | Anton Para | ||
| Sonicator | Grupo Selecta | 3000513 | 6 Litres. |
Referencias
- Scott, L. T., Hashemi, M. M., Bratcher, M. S. Corannulene bowl-to-bowl inversion is rapid at room temperature. J. Am. Chem. Soc. 114 (5), 1920-1921 (1992).
- Sygula, A., et al. Bowl stacking in curved polynuclear aromatic hydrocarbons: crystal and molecular structure of cyclopentacorannulene. J. Chem. Soc., Chem. Commun. (22), 2571-2572 (1994).
- Nuckolls, C., et al. Circular Dichroism and UV−Visible Absorption Spectra of the Langmuir−Blodgett Films of an Aggregating Helicene. J. Am. Chem. Soc. 120 (34), 8656-8660 (1998).
- Beljonne, D., et al. Electro-optic response of chiral helicenes in isotropic media. J. Chem. Phys. 108 (4), 1301-1304 (1998).
- Treboux, G., Lapstun, P., Wu, Z., Silverbrook, K. Electronic conductance of helicenes. Chem. Phys. Lett. 301 (5-6), 493-497 (1999).
- Katz, T. J. Syntheses of Functionalized and Aggregating Helical Conjugated Molecules. Angew. Chem., Int. Ed. 39 (11), 1921-1923 (2000).
- Furche, F., et al. Circular Dichroism of Helicenes Investigated by Time-Dependent Density Functional Theory. J. Am. Chem. Soc. 122 (8), 1717-1724 (2000).
- Urbano, A. Recent Developments in the Synthesis of Helicene-Like Molecules. Angew. Chem., Int. Ed. 42 (34), 3986-3989 (2003).
- Botek, E., Champane, B., Turki, M., André, J. M. Theoretical study of the second-order nonlinear optical properties of [N]helicenes and [N]phenylenes. J. Chem. Phys. 120 (4), 2042-2048 (2004).
- Lovas, F. J., et al. Interstellar Chemistry: A Strategy for Detecting Polycyclic Aromatic Hydrocarbons in Space. J. Am. Chem. Soc. 127 (12), 4345-4349 (2005).
- Wigglesworth, T. J., Sud, D., Norsten, T. B., Lekhi, V. S., Branda, N. R. Chiral Discrimination in Photochromic Helicenes. J. Am. Chem. Soc. 127 (20), 7272-7273 (2005).
- Wu, Y. -. T., Siegel, J. S. Aromatic Molecular-Bowl Hydrocarbons: Synthetic Derivatives, Their Structures, and Physical Properties. Chem. Rev. 106 (12), 4843-4867 (2006).
- Tsefrikas, V. M., Scott, L. T. Geodesic Polyarenes by Flash Vacuum Pyrolysis. Chem. Rev. 106 (12), 4868-4884 (2006).
- Wu, Y. -. T., Hayama, T., Baldrige, K. K., Linden, A., Siegel, J. S. Synthesis of Fluoranthenes and Indenocorannulenes: Elucidation of Chiral Stereoisomers on the Basis of Static Molecular Bowls. J. Am. Chem. Soc. 128 (21), 6870-6884 (2006).
- Wu, Y. -. T., Siegel, J. S. Synthesis, structures, and physical properties of aromatic molecular-bowl hydrocarbons. Top. Curr. Chem. 349, 63-120 (2014).
- Pérez, E. M., Martìn, N. Curves ahead: molecular receptors for fullerenes based on concave-convex complementarity. Chem. Soc. Rev. 37 (8), 1512-1519 (2008).
- Tashiro, K., Aida, T. Metalloporphyrin hosts for supramolecular chemistry of fullerenes. Chem. Soc. Rev. 36 (2), 189-197 (2007).
- Kawase, T. Ball- Bowl- and Belt-Shaped Conjugated Systems and Their Complexing Abilities: Exploration of the Concave−Convex π−π Interaction. Chem. Rev. 106 (12), 5250-5273 (2006).
- Martin, N., Pérez, E. M. Molecular tweezers for fullerenes. Pure Appl. Chem. 82 (3), 523-533 (2010).
- Hoppe, H., Sariciftci, N. S. Morphology of polymer/fullerene bulk heterojunction solar cells. J. Mater. Chem. 16 (1), 45-61 (2006).
- Kim, S. N., Rusling, J. F., Papadimitrakopoulos, F. Carbon Nanotubes for Electronic and Electrochemical Detection of Biomolecules. Adv. Mater. 19 (20), 3214-3228 (2007).
- Dennler, G., Scharber, M. C., Brabec, C. J. Polymer-Fullerene Bulk-Heterojunction Solar Cells. Adv. Mater. 21 (13), 1323-1338 (2009).
- Helgesen, M., Søndergaard, R., Krebs, F. C. Advanced materials and processes for polymer solar cell devices. J. Mater. Chem. 20 (1), 36-60 (2010).
- Brabec, C. J., et al. Polymer-Fullerene Bulk-Heterojunction Solar Cells. Adv. Mater. 22 (34), 3839-3856 (2010).
- Delgado, J. L., Bouit, P. -. A., Filippone, S., Herranz, M. A., Martìn, N. Organic photovoltaics: a chemical approach. Chem. Commun. 46 (27), 4853-4865 (2010).
- Schnorr, J. M., Swager, T. M. Emerging Applications of Carbon Nanotubes. Chem. Mater. 23 (3), 646-657 (2011).
- Wang, C., Takei, K., Takahashi, T., Javey, A. Carbon nanotube electronics - moving forward. Chem. Soc. Rev. 42 (7), 2592-2609 (2013).
- Park, S., Vosguerichian, M., Bao, Z. A review of fabrication and applications of carbon nanotube film-based flexible electronics. Nanoscale. 5, 1727-1752 (2013).
- Mizyed, S., et al. Embracing C60 with Multiarmed Geodesic Partners. J. Am. Chem. Soc. 123 (51), 12770-12774 (2001).
- Sygula, A., Sygula, R., Ellern, A., Rabideau, P. W. Novel Twin Corannulene: Synthesis and Crystal Structure Determination of a Dicorannulenobarrelene Dicarboxylate. Org. Lett. 5 (15), 2595-2597 (2003).
- Georghiou, P. E., Tran, A. H., Mizyed, S., Bancu, M., Scott, L. T. Concave Polyarenes with Sulfide-Linked Flaps and Tentacles: New Electron-Rich Hosts for Fullerenes. J. Org. Chem. 70 (16), 6158-6163 (2005).
- Sygula, A., Fronczek, F. R., Sygula, R., Rabideau, P. W., Olmstead, M. M. A Double Concave Hydrocarbon Buckycatcher. J. Am. Chem. Soc. 129 (13), 3842-3843 (2007).
- Yanney, M., Sygula, A. Tridental molecular clip with corannulene pincers: is three better than two?. Tetrahedron Lett. 54 (21), 2604-2607 (2013).
- Stuparu, M. C. Rationally Designed Polymer Hosts of Fullerene. Angew. Chem., Int. Ed. 52 (30), 7786-7790 (2013).
- Le, V. H., Yanney, M., McGuire, M., Sygula, A., Lewis, E. A. Thermodynamics of Host-Guest Interactions between Fullerenes and a Buckycatcher. J. Phys. Chem. B. 118 (41), 11956-11964 (2014).
- Álvarez, C. M. Enhanced association for C70 over C60 with a metal complex with corannulene derivate ligands. Dalton Trans. 43 (42), 15693-15696 (2014).
- Álvarez, C. M. Assembling Nonplanar Polyaromatic Units by Click Chemistry. Study of Multicorannulene Systems as Host for Fullerenes. Org. Lett. 17 (11), 2578-2581 (2015).
- Yanney, M., Fronczek, F. R., Sygula, A. A 2:1 Receptor/C60 Complex as a Nanosized Universal Joint. Angew. Chem. Int. Ed. 54 (38), 11153-11156 (2015).
- Kuragama, P. L. A., Fronczek, F. R., Sygula, A. Bis-corannulene Receptors for Fullerenes Based on Klärner's Tethers: Reaching the Affinity Limits. Org. Lett. 17 (21), (2015).
- George, S. R. D., Frith, T. D. H., Thomas, D. S., Harper, J. B. Putting corannulene in its place. Reactivity studies comparing corannulene with other aromatic hydrocarbons. Org. Biomol. Chem. 13 (34), 9035-9041 (2015).
- Shen, Y., Chen, C. -. F. Helicenes: Synthesis and Applications. Chem. Rev. 112 (3), 1463-1535 (2012).
- Crassous, J., Saleh, N., Shen, C. Helicene-based transition metal complexes: synthesis, properties and applications. Chem. Sci. 5 (10), 3680-3694 (2014).
- Nakamura, K., Furumi, S., Takeuchi, M., Shibuya, T., Tanaka, K. Enantioselective Synthesis and Enhanced Circularly Polarized Luminescence of S-Shaped Double Azahelicenes. J. Am. Chem. Soc. 136 (15), 5555-5558 (2014).
- Schweinfurth, D., Zalibera, M., Kathan, M., Shen, C., Mazzolini, M., Trapp, N., Crassous, J., Gescheidt, G., Diederich, F. Helicene Quinones: Redox-Triggered Chiroptical Switching and Chiral Recognition of the Semiquinone Radical Anion Lithium Salt by Electron Nuclear Double Resonance Spectroscopy. J. Am. Chem. Soc. 136 (37), 13045-13052 (2014).
- Šámal, M., Chercheja, S., Rybáček, J., Vacek Chocholoušová, J., Vacek, J., Bednárová, L., Šaman, D., Stará, I. G., Starý, I. An Ultimate Stereocontrol in Asymmetric Synthesis of Optically Pure Fully Aromatic Helicenes. J. Am. Chem. Soc. 137 (26), 8469-8474 (2015).
- Siegel, J. S., Butterfield, A. M., Gilomen, B. Kilogram scale production of corannulene. Organic Process Research & Development. 16 (4), 664-676 (2012).
- Mallory, F. B., Mallory, C. W. Photocyclization of stilbenes and related molecules. Organic Reactions. , (1984).
- Sato, M., et al. Convenient synthesis and reduction properties of [7] circulene. J. Chem. Soc., Perkin Trans. 2. (9), 1909-1914 (1998).
- Anderson, G. K., Lin, M. Bis(Benzonitrile)dichloro complexes of palladium and platinum. Inorg Synth. 28, 60-63 (1990).
- Nataro, C., Fosbenner, S. M. Synthesis and Characterization of Transition-Metal Complexes Containing 1,1'-Bis(diphenylphosphino)ferrocene. J. Chem. Ed. 86 (12), 1412-1415 (2009).
- Kauffman, G. B., Pinnell, R. P. Copper (I) Iodide. Inorg. Synth. 6, 3-6 (1960).
- Sonogashira, K. J. Development of Pd-Cu catalyzed cross-coupling of terminal acetylenes with sp2-carbon halides. Organomet. Chem. 653 (1-2), 46-49 (2002).
- Chinchilla, R., Nájera, C. Recent advances in Sonogashira reactions. Chem. Soc. Rev. 40 (10), 5084-5121 (2011).
- Kolb, H. C., Finn, M. G., Sharpless, K. B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 40 (11), 2004-2021 (2001).
- Spiteri, C., Moses, J. E. Copper-Catalyzed Azide-Alkyne Cycloaddition: Regioselective Synthesis of 1,4,5-Trisubstituted 1,2,3-Triazoles. Angew. Chem. Int. Ed. 49 (1), 31-33 (2010).
Reimpresiones y Permisos
Solicitar permiso para reutilizar el texto o las figuras de este JoVE artículos
Solicitar permisoThis article has been published
Video Coming Soon
ACERCA DE JoVE
Copyright © 2025 MyJoVE Corporation. Todos los derechos reservados
Utilizamos cookies para mejorar su experiencia en nuestra página web.
Al continuar usando nuestro sitio web o al hacer clic en 'Continuar', está aceptando nuestras cookies.