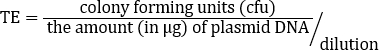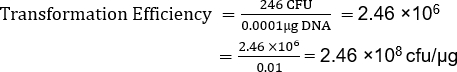Transformation of E. coli Cells Using an Adapted Calcium Chloride Procedure
Source: Natalia Martin1, Andrew J. Van Alst1, Rhiannon M. LeVeque1, and Victor J. DiRita1
1 Department of Microbiology and Molecular Genetics, Michigan State University
Bacteria have the ability to exchange genetic material (DeoxyriboNucleic Acid, DNA) in a process known as horizontal gene transfer. Incorporating exogenous DNA provides a mechanism by which bacteria can acquire new genetic traits that allow them to adapt to changing environmental conditions, such as the presence of antibiotics or antibodies (1) or molecules found in natural habitats (2). There are three mechanisms of horizontal gene transfer: transformation, transduction, and conjugation (3). Here we will focus on transformation, the ability of bacteria to take up free DNA from the environment. In the laboratory, the transformation process has four general steps: 1) Preparation of competent cells, 2) Incubation of competent cells with DNA, 3) Recovery of cells, and 4) Plating of the cells for growth of the transformants (Figure 1).

Figure 1: General steps of the transformation process. The transformation process has four general steps: 1) Preparation of competent cells, 2) Incubation with DNA, 3) Recovery of the cells and 4) Plating cells for growth of the transformants.
For transformation to occur, the recipient bacteria must be in a state known as competence. Some bacteria have the ability to become naturally competent in response to certain environmental conditions. However, many other bacteria do not become competent naturally, or the conditions for this process are yet unknown. The ability to introduce DNA into bacteria has a range of research applications: to generate multiple copies of a DNA molecule of interest, to express large amount of proteins, as a component in cloning procedures, and others. Because of the value of transformation to molecular biology, there are several protocols aimed to make cells artificially competent when conditions for natural competence are unknown. Two main methods are used to prepare artificially competent cells: 1) through chemical treatment of cells and 2) exposing the cells to electric pulses (electroporation). The former uses different chemicals depending on the procedure to create attraction between the DNA and the cell surface, while the latter uses electric fields to generate pores in the bacterial cell membrane through which DNA molecules can enter. The most efficient approach for chemical competence is incubation with divalent cations, most notably calcium (Ca2+) (4,5) Calcium-induced competence is the procedure that will be described here (6). This method is mainly used for transformation of Gram-negative bacteria, and that will be the focus of this protocol.
The procedure of chemical transformation involves a series of steps in which cells are exposed to cations to induce chemical competence. These steps are subsequently followed by a temperature change - heat shock - that favors the uptake of foreign DNA by the competent cell (7). The bacterial cell envelopes are negatively charged. In Gram-negative bacteria like Escherichia coli, the outer membrane is negatively-charged due to the presence of lipopolysaccharide (LPS) (8). This results in repulsion of the similarly negatively-charged DNA molecules. In chemical competence induction, positively-charged calcium ions neutralize this charge repulsion enabling DNA absorbance onto the cell surface (9). Calcium treatment and incubation with DNA are done on ice. Subsequently, an incubation at higher temperatures (42°C), the heat shock, is performed. This temperature imbalance further favors the DNA uptake. Bacterial cells need to be at the mid-exponential growth phase to withstand the heat shock treatment; in other growth stages the bacterial cells are too sensitive to the heat resulting in loss of viability which significantly decreases transformation efficiency.
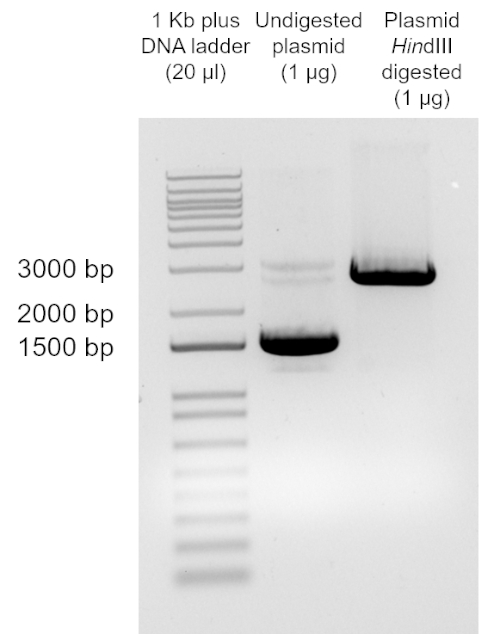
Different DNA sources can be used for transformation. Typically, plasmids, small circular, double-stranded DNA molecules, are used for transformation in most laboratory procedures in E. coli. For plasmids to be maintained in the bacterial cell after transformation, they need to contain an origin of replication. This allows them to be replicated in the bacterial cell independently from the bacterial chromosome. Not all the bacterial cells get transformed during the transformation procedure. Thus, transformation yields a mixture of transformed cells and non-transformed cells. To distinguish between these two populations, a selection method to identify the cells that have acquired the plasmid is used. Plasmids usually contain selectable markers, which are genes encoding a trait that confers an advantage for growth (i.e. resistance to an antibiotic or chemical or rescue from a growth auxotrophy). After transformation, bacterial cells are plated on selective media, which only allows for growth of the transformed cells. In the case of cells transformed with a plasmid conferring resistance to a given antibiotic, the selective media will be growth media containing that antibiotic. Several different methods can be used to confirm that the colonies grown in the selective media are transformants (i.e. have incorporated the plasmid). For example, plasmids can be recovered from these cells using plasmid preparation methods (10) and digested to confirm plasmid size. Alternatively, colony PCR can be used to confirm the presence of the plasmid of interest (11).
The aim of this experiment is to prepare E. coli DH5α chemically competent cells, using an adaptation of the calcium chloride procedure (12), and to transform them with the plasmid pUC19 to determine transformation efficiency. The E. coli strain DH5α is a commonly used strain in molecular biology applications. Because of its genotype, specifically the recA1 and endA1, this strain allows for increased insert stability and improve the quality of plasmid DNA in subsequent preparations. Since the transformation efficiency decreases with increasing size of the DNA, the plasmid pUC19 was used in this protocol because of its small size (2686 bp) (see https://www.mobitec.com/cms/products/bio/04_vector_sys/standard_cloning_vectors.html for a vector map). pUC19 confers resistance to ampicillin and thus, this was the antibiotic used for selection.
This protocol describes the preparation and transformation of competent E. coli DH5α using an adaptation of the calcium chloride procedure (12).
1. Set-up
- Equipment
- Spectrophotometer
- Sorval Centrifuge (or equivalent)
- Benchtop centrifuge
- Heat block or water bath
- Orbital Shaker
- Stationary Incubator
- Gel casting tray
- Well combs
Although TE is dependent on many factors, non-commercial competent cell preparations, like this one, normally yield 106 to 107 transformants per microgram of plasmid. Therefore, this preparation, with a TE = 2.46 x 108 cfu/µg, yielded a TE well beyond the expected range. Additional protocols are available for making supercompetent cells when higher transformation efficiencies are required for a given application (13).
Log in or to access full content. Learn more about your institution’s access to JoVE content here
Transformation is a powerful method for introducing exogenous DNA into bacterial cells that is key to many molecular biology applications in the laboratory. Additionally, it plays a major role in nature by allowing bacterial cells to exchange genetic material that could result in increased genetic variation and allow for acquisition of different beneficial traits for survival under a wide range of conditions. Many bacterial strains encode the genes required for natural competence. However, the conditions in which these g
- Croucher, N. J. et al. Rapid pneumococcal evolution in response to clinical interventions. Science. 331 (6016):430-434. (2011)
- Borgeaud, S. et al. The type VI secretion system of Vibrio cholerae fosters horizontal gene transfer. Science. 347(6217):63-67. (2015)
- Burmeister, A. R. Horizontal Gene Transfer. Evol Med Public Health. 2015 (1):193-194. (2015)
- Weston A, Brown MG, Perkins HR, Saunders JR, Humphreys GO. Transformation of Escherichia coli with plasmid deoxyribonucleic acid: calcium-induced binding of deoxyribonucleic acid to whole cells and to isolated membrane fractions. J Bacteriol. 145 (2):780-7. (1981)
- Dagert M, Ehrlich SD. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 6 (1):23-8. (1979)
- Asif A, Mohsin H, Tanvir R, and Rehman Y. Revisiting the Mechanisms Involved in Calcium Chloride Induced Bacterial Transformation. Front Microbiol. 8:2169. (2017)
- Panja S, Aich P, Jana B, Basu T. How does plasmid DNA penetrate cell membranes in artificial transformation process of Escherichia coli? Mol Membr Biol. 25 (5):411-22. (2008)
- Silhavy, TJ, Kahne D, Walker S. The Bacterial Cell Envelope. Cold Spring Harb Perspect Biol. 2 (5): a000414. (2010)
- Panja S, Aich P, Jana B, Basu T. (2008) Plasmid DNA binds to the core oligosaccharide domain of LPS molecules of E. coli cell surface in the CaCl2-mediated transformation process. Biomacromolecules. 9 (9):2501-9.
- JoVE Science Education Database. Basic Methods in Cellular and Molecular Biology. Plasmid Purification. JoVE, Cambridge, MA. (2018)
- Bergkessel M and Guthrie C. Colony PCR. Methods in Enzymology. 529: 299-309. (2013)
- Sambrook J and Russell DW. Molecular Cloning A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.Protocol 25 (1.116-118). (2001)
- Wirth R, Friesenegger A, Fiedler S. Transformation of various species of gram-negative bacteria belonging to 11 different genera by electroporation. Molecular & General Genetics. 216 (1): 175-7. (1989)
Saltar a...
Vídeos de esta colección:

Now Playing
Transformation of E. coli Cells Using an Adapted Calcium Chloride Procedure
Microbiology
85.8K Vistas

Creación de la columna de Winogradsky: Un método que sirve para enriquecer las especies microbianas en una muestra de sedimento
Microbiology
127.1K Vistas

Diluciones en serie y enchapado: enumeración microbiana
Microbiology
311.6K Vistas

Cultivos de enriquecimiento: Cultivo de bacterias aerobias y anaerobias en medios selectivos y diferenciales
Microbiology
131.2K Vistas

Cultivos puros y siembra por estrías: aislamiento de colonias bacterianas únicas de una muestra mixta
Microbiology
164.9K Vistas

Secuenciación del ARNr 16s: Una técnica basada en PCR para identificar especies bacterianas
Microbiology
186.6K Vistas

Curvas de crecimiento: Generación de curvas de crecimiento utilizando unidades formadoras de colonias y mediciones de densidad óptica
Microbiology
289.8K Vistas

Pruebas de susceptibilidad a los antibióticos: Pruebas con epsilometro para determinar los valores de la CMI de dos antibióticos y evaluar la sinergismos
Microbiology
93.1K Vistas

Microscopía y tinción: Tinción de Gram, cápsula y endosporas
Microbiology
361.4K Vistas

Ensayo de placa: Un método para determinar los títulos virales como unidades formadoras de placas (UFP)
Microbiology
184.9K Vistas

Conjugación: Un método para transferir resistencia a ampicilina de un donante a una E. Coli receptora
Microbiology
37.8K Vistas

Transducción de fagos: Un método para transferir resistencia a ampicilina de un donante una E. coli receptora
Microbiology
28.7K Vistas
ACERCA DE JoVE
Copyright © 2025 MyJoVE Corporation. Todos los derechos reservados