1. planification préopératoire
bien que les lignes directrices n’exigent pas que les rongeurs chirurgies être exécutée dans un établissement chirurgical dédié, la superficie utilisée doit être désinfectée avec une surface dure appropriée désinfectant, qui doit être utilisé selon le fabricant ' s énumérés les concentrations et les temps de contact. La région devrait aussi être exempts de tout encombrement et ne pas être dans la ligne directe de l’alimentation et d’échappement conduits, comme les projets pourraient contribuer à l’hypothermie de l’animal. Accès à la salle devrait être limitée lorsque les interventions chirurgicales sont effectuées. Une zone de préparation chirurgicale (notamment pour l’enlèvement de l’animal ' cheveux s) et pour récupération post-opératoire et soins, devrait également être désignée et à proximité, sinon dans la salle de chirurgie. En général, si les interventions chirurgicales sont effectuées régulièrement dans un domaine spécifique, la zone ne devrait pas servir pour d’autres activités.
préparations préopératoires devraient comprendre un examen physique du patient chirurgical pour identifier toute affections sous-jacentes qui peuvent interférer avec la chirurgie. Puisque les rongeurs ont un taux métabolique élevé, et les réserves de graisse très limitée, ils ne devraient pas être jeun avant la chirurgie. L’animal ' l’état d’hydratation s devrait être évaluée par un test d’élasticité de la peau. La peau au-dessus des épaules est légèrement levée. Chez un animal normalement hydraté, la peau rapidement reviendra en place, alors que chez des animaux déshydraté, la peau pas immédiatement revenir à sa position normale. Dans l’ensemble apparence, tels que la posture et l’état de la couche de poils, convient également de noter. Un animal qui affiche une posture voûtée, ou a un pelage hirsute, peut être héberger une maladie. L’examen doit être effectué juste avant l’administration de l’anesthésie, et toute condition anormale doit être notée sur l’animal ' graphique s. 2 Enfin, considérations relatives à l’anatomie et la physiologie du rat ou de souris doivent se faire lorsque les préparer à la chirurgie.
2. plateforme
les deux espèces ont une surface importante au rapport de volume de corps ce qui les rend sensibles à l’hypothermie lors d’interventions chirurgicales, surtout quand la chirurgie expose la cavité du corps. Efforts pour éviter l’hypothermie incluent l’utilisation d’une plateforme chirurgicale chauffée.
la plate-forme utilisée pour la chirurgie de rongeur, qui n’est généralement construite d’acier inoxydable ou en plastique dur, doit être recouverte d’un matériau isolant ou une source de chauffage d’appoint pour empêcher l’animal ' chaleur corporelle s de s’échapper pendant la mode opératoire. Chauffage d’appoint sources inclure l’eau en circulation chauffage tampons, forcés chauffe-air couvertures, lampes chauffantes ou une couche de mousse couvrant la plate-forme chirurgicale. Plates-formes chirurgicales avec construit dans la source de chaleur sont disponibles dans le commerce. Toutes les plates-formes doivent être d’un matériau qui est facilement désinfectée et insensible à l’humidité.
autres méthodes pour prévenir l’hypothermie comprennent l’utilisation d’isolateurs massives, placement isolants entre l’animal et la plate-forme chirurgicale et à l’aide d’une source de chaleur externe. 3 isolateurs de masse piéger air dans une matrice de fibre, produisant " encore air " qui entoure l’animal. Couvertures de l’eau chaude en circulation peut être utilisé sous le patient. Cet équipement est disponible dans diverses tailles de rongeurs d’équin et permet de soutien thermique précis avec thermostats intégrés.
chimiquement activés sources de chauffage peut être à usage unique ou réutilisables. Un type se compose d’une poche en plastique remplie avec une solution chimique et une rondelle de métal, qui, une fois pressé crée une réaction exothermique. Cela provoque le liquide à solidifier et à libérer la chaleur. En règle générale, ils ont une quantité limitée de la chaleur et conviennent uniquement pour les procédures rapides. Autres sources de chauffage chimique sont disponibles sous forme de solides à température ambiante, mais lorsqu’il est chauffé, ils deviennent liquides. Qu’un animal est placé sur le pad, le liquide libère chaleur et le contenu du tampon se solidifie qu’ils refroidissent. Ceux-ci peuvent libérer la chaleur sur une période beaucoup plus longue. Comme un avantage, ils ne doit pas dépasser la température d’activation (~ 39° C), éliminant ainsi la nécessité d’un thermostat de.
packs d’eau sont disponibles en bouteilles d’eau chaude consistant en un sac de caoutchouc ou de silicone avec un bouchon. Les packs sont remplis avec de l’eau chaude puis émet de la chaleur sur la surface extérieure. Le pack perdront graduellement la chaleur que l’eau se refroidit. Une version plus moderne se compose d’une matière plastique feuille avec tissu perméable à l’eau ont adhéré au sommet. L’espace entre les deux est rempli d’une poudre hydrophile qui absorbe l’eau et gonfle. Il peut être utilisé soit une source de chaleur ou comme une source de refroidissement. Selon la qualité des matériaux, il ou peut être recyclé et réchauffé au micro-ondes trempée dans l’eau chaude.
précautions doivent être prises lorsque vous utilisez une source de chaleur externe. Température corporelle doit être surveillée avec une sonde rectale ou avec un thermomètre placé à côté de l’animal sur la source de chaleur. Toutes les sources de chaleur externes doivent être vérifiées pour les défauts avant utilisation.
3. Épilation des poils
du site chirurgical doit être préparé pour minimiser la contamination de l’incision. Les cheveux doivent être étroitement coupé ou supprimé avec une crème dépilatoire chimique, qui dissout le poil dans le follicule. 2, 3 bien que le détourage de cheveux parfois peut être effectué que sur un animal conscient avec retenue manuelle, l’application de la crème dépilatoire ne doit se faire sur un animal anesthésié pour prévenir l’ingestion du produit, des lésions oculaires et enlèvement des poils excessifs. Rasage avec un rasoir est une option, s’il n’y a pas d’alternative. Cette méthode requiert des compétences techniques, le temps supplémentaire et patience afin d’éviter des lacérations à la peau. Le champ opératoire doit être suffisamment grand pour permettre d’incision et de suture sans inclusion de la fourrure dans la plaie opératoire, mais aussi petit que possible de manière à éviter l’aggravation de l’hypothermie.
- De tonte cheveux peut être coupé à l’aide de tondeuses électriques sur secteur ou à piles, de préférence avec une lame chirurgicale de l’A40. La largeur de la lame doit être considérée. Une norme 2 " lame peut être utilisée pour les rats, alors qu’un ½-1 " des lames est plus approprié pour les souris.
- Les cheveux est découpé dans le sens inverse de la croissance. Tendez la peau pour stabiliser, comme les rongeurs ont une fixation lâche de la peau vers le muscle sous-jacent.
- Il faut éviter les entailles ou couper la peau. L’extrémité plate de la lame est placée sur la peau lorsque tondre le cheveux. La lame ne doit jamais être utilisée avec les dents perpendiculaires à la peau.
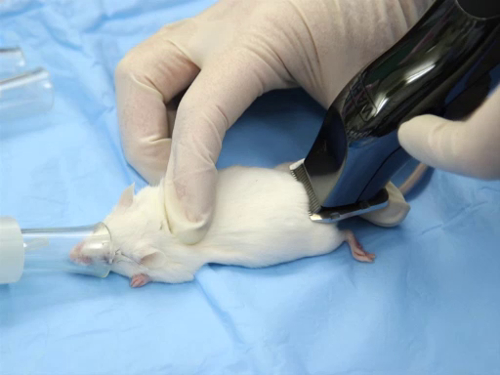
figure 1. La position correcte d’une tondeuse à cheveux pendant le rasage.
- les crèmes dépilatoires chimiques ou des lotions
- appliquer le produit sur la zone opératoire.
- Bout de 10 minutes, la peau doit être complètement rincernettoyé de toute trace de l’épilation pour éviter les brûlures irritations ou de produit chimique sur la peau et de d.
4. Chirurgien
solutions de chirurgien à utiliser doivent : 1) sensiblement réduire les microbes présents sur la peau et contiennent une préparation antimicrobienne non irritant ; 2) possède un large spectre des propriétés antimicrobiennes ; 3) être à action rapide ; et 4) ont une activité persistante, cumulatif.
les deux solutions de gommage nettoyant couramment utilisés sont les chlorhexidines et les iodophores. Solutions de chlorhexidine sont efficaces contre les bactéries et les virus, même en présence de matière organique. En revanche, les iodophores ont un large éventail d’action microbicide, mais leur efficacité est réduite en présence de matières organiques ; l’activité résiduelle est inférieure à celle de la chlorhexidines.
rinçages utilisés entre les gommages sont soit de l’eau stérile ou de l’alcool. Les solutions à base d’alcool contenant 60-95 % d’alcool ont grandes actions antimicrobiens au travers de la dénaturation des protéines. 2 Toutefois, l’alcool peut être un irritant de la peau forte. Eau stérile est efficace dans la zone de rinçage, et pourtant il n’a pas toutes les propriétés antimicrobiennes.
- Utiliser un tampon de gaze humide pour enlever les débris brut, y compris les poils et squames.
Solution de nettoyage antiseptique - imbibée sur un tampon de gaze est appliquée sur la peau à partir du site d’incision. Désinfection devrait commencer le long de la ligne d’incision et s’étendent vers l’extérieur dans un motif circulaire.
- L’antiseptique rincer est ensuite appliqué en commençant à l’emplacement de l’incision. La peau est essuyée dans un motif circulaire en spirale vers la périphérie du champ chirurgical pour enlever la solution de nettoyage de la ligne d’incision.
- Cela est répété trois fois.
- Après un rinçage final, une compresse de gaze stérile est placée au-dessus du champ opératoire. Cette gaze peut être mouillée avec l’alcool ou d’iode. La gaze disparaît une fois que l’animal est transporté et mis en position sur une plateforme chirurgicale ou de la table/banc chirurgicale.
des zones du corps où les méthodes de lavage chirurgicales standards ne servent pas incluent les yeux, la bouche et la région anale. Parce que la surface de le œil serait endommagée si gommage solutions ont été utilisées, un chirurgien se fait uniquement sur les paupières après inculquer une pommade protectrice dans le œil. Dans certaines situations, une solution saline physiologique équilibrée est utilisée pour rincer l’oeil pour enlever les débris et de diluer des bactéries à un niveau acceptable pour une chirurgie de se produire. La bouche aussi s’avère difficile à nettoyer suffisamment pour la chirurgie. Il peut être rincé avec une solution saline physiologique équilibrée pour diluer toutes les bactéries ; Toutefois, il est important d’éviter d’utiliser trop saline, qui pourrait causer l’aspiration du liquide. Gencives, dents et la langue peuvent être essuyées avec un non toxique antiseptiques. Cependant, l’application de solutions pour les muqueuses peut entraîner une absorption systémique. Interventions chirurgicales dans la région anale, telles que la réduction chirurgicale du prolapsus rectales, ne sont pas considérés comme des chirurgies propres. L’utilisation de certaines solutions antiseptiques peut augmenter les lésions tissulaires et prévenir ou prolonger la guérison. L’utilisation d’une solution saline physiologique équilibrée pour laver la zone propre de débris est la méthode préférée de préparation chirurgicale. 2, 4
5. Positionnement
positionnement du Patient pour les procédures abdominales implique la sécurisation des membres de l’enclin animal sur la plate-forme avec un ruban ou une ligature. Lorsque vous utilisez une ligature pour étendre les branches, il faut pour empêcher la circulation vers les pieds étant compromise, afin d’éviter une tension excessive sur les branches et un étirement extrême des membres susceptibles de perturber les articulations et d’éviter l’obstacle de la respiration. Les liens devraient être un dégagement rapide avec seulement une boucle demi-clef sur la branche. Certaines plates-formes disponibles dans le commerce viennent avec rétraction limb intégré qui se compose des crochets ou des boucles de fil d’acier inoxydable ou chaîne de boule, qui peut être ajustée selon la taille de l’animal. Si le ruban est utilisé, il faut se conformer aux surfaces sèches.
6. drapage
une fois que l’animal est préparée et placé sur la plate-forme chirurgicale, draps chirurgicaux sont utilisés pour prévenir la contamination du matériel de suture et de maintenir un champ stérile sur le site chirurgical. Draperies peuvent être un matériel de tissu réutilisables, un matériel de papier jetable ou un matériau adhésif en plastique jetable.
rideaux papier jetables ont une matrice de fibres tissées pour la force qui permet de couper dans toutes les formes et dimensions, y compris une fenestration de coupe ou d’ouverture dans le drapé, sans déchirure ni l’effilochage des bords coupés. Ils sont également l’humidité répulsifs. Les draps jetables peuvent être achetés préemballés et préstérilisé dans une variété de tailles et formes. Tissu rideaux n’est pas conçus pour être coupé par le chirurgien pour créer une fenestration. Ils sont achetés avec une fenestration précoupés et lié le bord. Tissu rideaux nécessite blanchiment d’argent et de stérilisation. Lorsque pris en charge bien, rideaux de tissu peut durer pendant des années, ce qui les rend un investissement économique.
tous les deux en papier et tissu rideaux est maintenus en place avec des pinces de serviette à travers la peau de l’animal si c’est un rongeur plus gros, comme un rat adulte. Pour petits rongeurs, le drapé n’est pas apposé sur la peau, ce qui nécessite une vigilance et des soins de la part du chirurgien de ne pas déloger ou décaler le drap une fois qu’il a été positionné sur l’animal.
adhésifs tentures sont soit transparent ou opaque. Les rideaux clairs sont préférables pour les chirurgies de rongeurs, car ils permettent la visualisation directe de l’animal. Des rideaux en plastique sont une combinaison de plastique et papier, avec la zone plastique étant directement au-dessus de l’animal et la zone de document définissant le champ stérile étendues. La portion du drapé qui soit directement sur le site de l’incision chirurgicale est conçue pour adhérer à la zone de l’incision. Le chirurgien peut ensuite couper directement à travers le plastique lors de l’incision cutanée. Pellicule plastique stérilisé a été accepté comme un matériau rentable et utile pour les chirurgies de rongeurs. Il faut éviter une constriction du mouvement pour la respiration lorsque l’écharpe est placé autour du patient. L’écharpe conserver la chaleur corporelle, permettra la visualisation du patient et fournissent une barrière d’humidité entre le champ stérile et l’animal. Il peut également servir à faciliter le positionnement et la tenue de l’animal pour la chirurgie au lieu de fixation limb.
rideaux de n’importe quel type devrait être soigneusement plié pour éviter le contact avec les zones non stériles, l’équipement et du personnel ; ils ne devraient jamais se sont déroulés en secouant ou en agitant.
- Rideaux papier : dans le drapé de méthode unique, le drapé est déplié pour permettre la découpe de la fenestration si on n’est pas prédécoupé.
- Le chirurgien placera le drapé sur l’animal en gardant les mains sur le côté de la draperie qui ne touchera pas le patient.
- Le drapé est ajusté afin que le champ opératoire est visible à travers la fenestration.
- Le drapé est maintenu en place avec des colliers de serviette à travers la peau de l’animal dans le cas de plus gros rats.
- Papier ou en plastique avec une fenêtre adhésive
- un drapé avec un adhésif fenêtre nécessite peeling de la zone de papier à la région d’adhésif à coller sur le champ chirurgical.
- Lorsque le déplier le drap stérile, la zone adhésive est généralement la région sommitale et est facilement accessible au chirurgien.
- Une fois que l’adhésif est découvert, le drapé est soigneusement déplié et tourné afin que le côté collant fait face à l’animal.
- Il est impératif que le drap est monté correctement, car une fois que l’adhésif entre en contact avec l’animal il ne pourra pas être ajusté.
- Le chirurgien devrait genTLY Appuyez sur l’adhésif du champ opératoire pour créer un joint avec la peau.
- Tissu rideaux
- fenêtrée tissu rideaux ne doivent ne pas être coupés, donc c’est le chirurgien ' responsabilité s pour sélectionner un drapé avec une ouverture suffisamment grande pour exposer correctement la zone chirurgicale, mais pas trop grand pour permettre l’exposition de le quelconque les surfaces du corps Unshaved et mal préparés.
- Le drapé est soigneusement déplié pour révéler la fenestration.
- Le chirurgien placera le drapé sur l’animal, garder les mains sur le côté de la draperie qui ne touchera pas le patient.
- Le drapé est ajusté afin que le champ opératoire est visible à travers la fenestration.
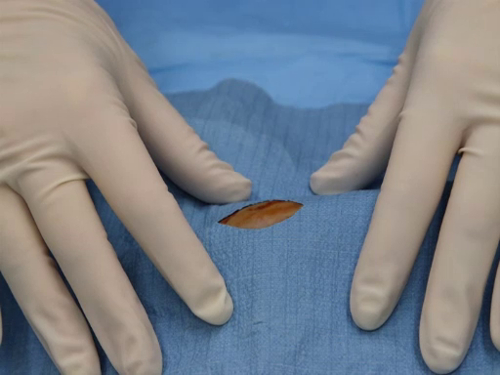
figure 2. Champ opératoire visible à travers la fenestration d’un drapé correctement placé.
- adhésif rideaux : un matériau économique et utile pour rongeurs chirurgies. 5
- drapé doit être tiré à partir du rouleau de manière à s’assurer que la section utilisée demeure stérile.
- L’assistant chirurgical ouvre la boîte et tire une longueur d’écharpe dehors, en prenant soin d’éviter de toucher à la boîte ou toute autre surface.
- Le chirurgien saisit l’enveloppe de chaque côté, et l’assistant coupe la fin ils tiennent loin (environ 3-4 pouces).
- Après avoir jeté le bord coupé, l’assistant recoupe aussi la section du reste de la course au.
- Le chirurgien saisit un côté de l’écharpe et le place sur l’animal.
- Les propriétés de l’écharpe lui permettent de se conformer à toutes les surfaces.
- Le chirurgien appuie sur le film à l’animal et crée un champ stérile.
- Il n’y a pas besoin d’utiliser des pinces serviette ou s’inquiéter que le drapé se déplace.
- Il faut éviter la constriction du mouvement pour la respiration. L’écharpe conservera la chaleur du corps, permettre la visualisation du patient et fournissent une barrière d’humidité entre le champ stérile et l’animal.
7. Monitorage peropératoire
Anesthetized patients doivent être surveillés pour la température du corps, respiration et rythme cardiaque jusqu'à ce qu’ils sont entièrement recouvrés.
température corporelle peut être contrôlée directement ou indirectement. Pour une surveillance directe, une sonde rectale, spécialement conçu pour les rongeurs doit être utilisée. Petits animaux thermomètres rectaux, mercure ou numérique, sont trop gros pour une utilisation dans des souris et des rats sans endommager les tissus rectales et le sphincter anal. Dans les souches sensibles, leur usage pourrait précipiter un prolapsus rectal. Surveillance indirecte consiste à placer un thermomètre à côté de l’animal ou sous le corps sur une source de chaleur externe. Bien que cela ne donnera pas une température exacte, il peut indiquer l’efficacité de la source de chaleur et permettre des ajustements réduire ou augmenter la chaleur selon les besoins.
il est difficile d’ausculter la fréquence cardiaque et de compter les respirations sur petits rongeurs sans équipement spécialisé.
surveillance plupart est visuelle et n’affiche plus que la présence ou l’absence de poitrine ou de la respiration abdominale. Fréquences cardiaques sont évalués comme présent ou absent par palpation ou l’observation visuelle du mouvement fine de la paroi thoracique. Ce n'est pas possible pendant une intervention chirurgicale en raison de drapage et la petite taille de l’animal.
une surveillance supplémentaire est possible grâce à l’utilisation des électrocardiogrammes (ECG) et oxymètres. ECG ' s évaluer l’état cardiaque d’un rongeur pendant l’anesthésie et la chirurgie. L’oxymètre de pouls utilise réfraction de lumière rouge et infrarouge pour mesurer l’oxygène dans le sang artériel. Cette technologie a été adaptée pour une utilisation chez les rongeurs à l’aide de la queue ou une patte. Les deux types de non invasives mesures continues du patient ' s les signes vitaux sont facilement accessibles avec un minimum de perturbations du champ chirurgical.
8. surveillance postopératoire
un devez envisager d’utiliser un coussin chauffant sous le postopératoires de cage de récupération. En outre, l’analgésie préventive et postopératoire devrait bénéficier autant que possible. Dispositions de l’analgésie sont plus efficaces pour réduire l’intensité de la stimulation douloureuse lorsqu’il est administré avant l’événement douloureux. Avantages de l’utilisation préventive des analgésiques incluent la réduction de l’intensité de la stimulation douloureuse, amélioration de l’animal ' postopératoires niveau de confort s, la réduction du montant nécessaire pour maintenir un plan chirurgical, l’anesthésie et un récupération plus lisse de l’anesthésie, une fois la procédure terminée. Médicament préventif et postopératoires fréquemment utilisés est indiqués dans le tableau 1. 6
| classe de médicament | nom | posologie | Fréquence |
| Anti-inflammatoire Non stéroïdien (Substance agerie) | kétoprofène | souris 2-5 mg/kg SC
5 mg/kg SC rats | tous les 12 – 24 heures
tous les 12 – 24 heures |
| Non-steroidal anti-i es médicaments (Substance agerie) | flunixine méglumine | 2,5 mg/kg SC souris | tous les 12 – 24 heures |
| anti-inflammatoire Non stéroïdien (Substance agerie) | Meloxicam | 5-10 mg/kg PO souris ou souris 1-2 mg/kg SC
5-10 mg/kg PO ou 1-2 mg/kg SC ou PO rat | tous les 12 – 24 heures
toutes les 24 heures
tous les 12 – 24 heures
toutes les 24 heures |
| anti-inflammatoire Non stéroïdien (Noncontrolle d Substance) | acétaminophène | 50 mg/kg SC/IP ou 100 mg/kg PO rats | tous les 8 – 12 heures |
| opioïde (Substance contrôlée) | Butorphanol | 0,5 à 3,0 mg/kg SC ou 0,2-2 mg/kg souris IP
2,0 mg / kg SC
0,2-2 mg/kg des rats IP | toutes les 4 heures
tous les 2 – 4 heures
toutes les 4 heures
tous les 2 – 4 heures |
| opioïde (Substance contrôlée) | buprénorphine | 0,05 à 2,5 mg/kg SC ou IP souris
0,01 – rat de 0,5 mg/kg SC | tous les 6 – 12 heures
tous les 8 – 12 heures |
| opioïde (Substance contrôlée) | Oxymorphone | 0,2 à 0,5 mg/kg SC souris
0,2 à 0,5 mg/kg SC rats | tous les 6 – 1 2 heures
tous les 6 – 12 heures |
Table 1. Couramment utilisé des drogues préventives et postopératoires.
















