16.15 : 酸塩基滴定曲線
滴定曲線とは、溶液のpHと滴定液の添加量をプロットしたものです。酸塩基滴定の場合、溶液の組成に応じたpHの変化が予測可能なため、滴定の進行状況や終点の検出をモニターするのに有用です。酸塩基滴定は、強酸と強塩基、強酸と弱塩基、または強塩基と弱酸で行うことができます。
0.100 MのHCl(強酸)25.00 mLを0.100 MのNaOH(強塩基)で滴定した場合、その滴定曲線はFigure 1aのように赤色で表示されます。また、0.100 MのCH3COOH(弱酸)25.00 mLを0.100 MのNaOHで滴定すると、その滴定曲線はFigure 1bのように黄色で表示されます。
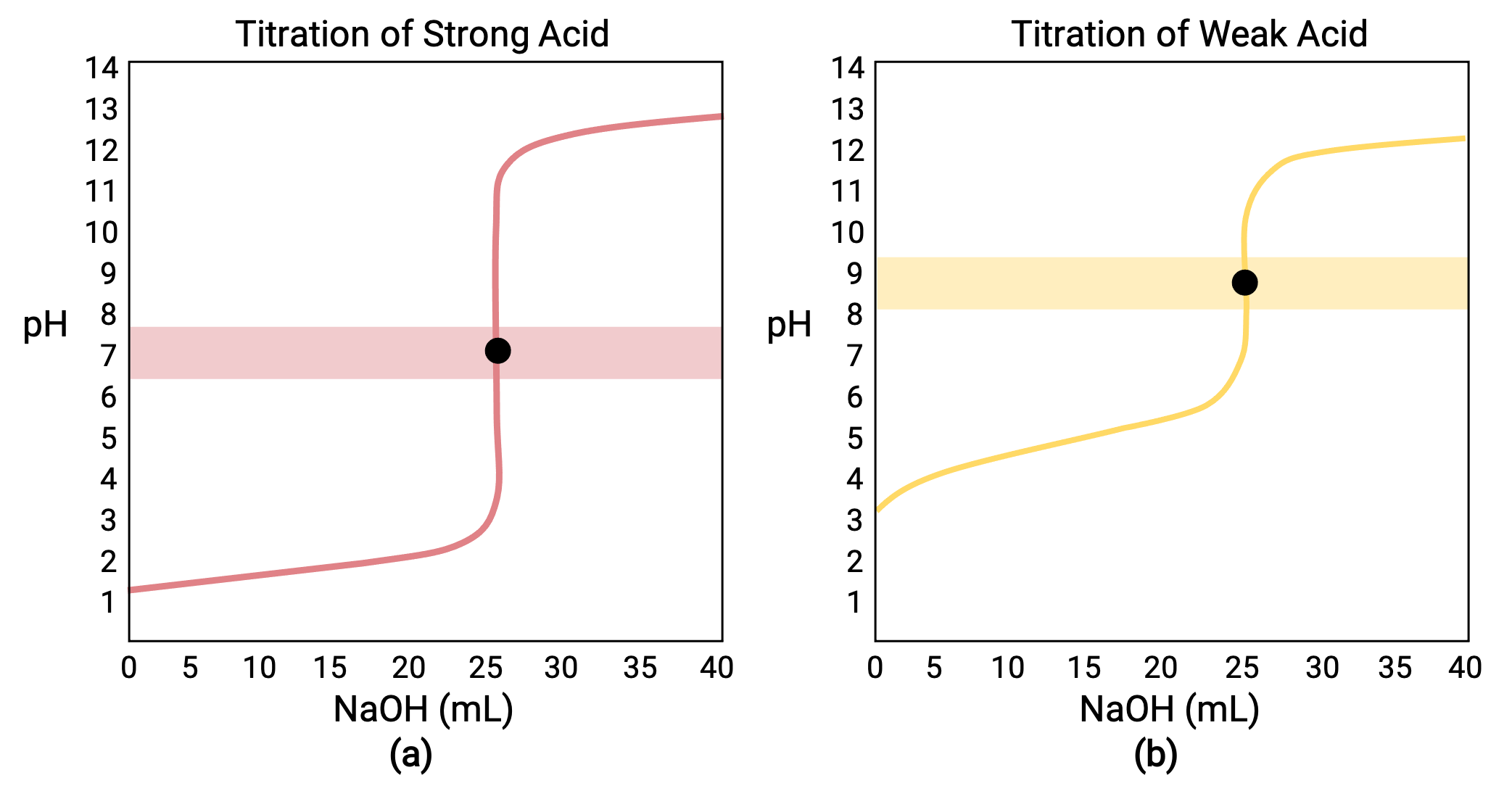
Figure 1 (a) 0.100 M塩酸(強酸)25.00 mLを0.100 M NaOH(強塩基)で滴定したときの滴定曲線は、当量点がpH7.00となります。(b) 0.100 M酢酸(弱酸)25.00 mLを0.100 M NaOH(強塩基)で滴定したときの滴定曲線の当量点は、pH8.72です。
滴定における4つの段階
- 初期状態(滴定液添加量 = 0mL): pHは、滴定する酸によって決まります。強酸と弱酸の酸が同濃度の場合、弱酸がより大きな初期pHを示します。
- 等量点前(0mL < V < 25 mL): 溶液のpHが徐々に上昇し、添加した滴定剤との反応で酸が消費され、組成には未反応の酸とその反応生成物である共役の塩基が含まれます。
- 等量点(V = 25 mL): 溶液の組成が酸性から中性(強酸を滴定する場合)または塩基性(弱酸を滴定する場合)に移行する際に、pHの急激な上昇が観察され、酸の共役塩基のイオン化によってpHが決まります。
上記の文章は以下から引用しました。 Openstax, Chemistry 2e, Section 14.7: Acid-base Titrations.
章から 16:

Now Playing
16.15 : 酸塩基滴定曲線
酸塩基と溶解度平衡
126.5K 閲覧数

16.1 : 共通イオン効果
酸塩基と溶解度平衡
41.0K 閲覧数

16.2 : 緩衝液
酸塩基と溶解度平衡
163.5K 閲覧数

16.3 : ヘンダーソン-ハッセルバルヒ式
酸塩基と溶解度平衡
68.2K 閲覧数

16.4 : 緩衝液中のpHの変化の計算
酸塩基と溶解度平衡
52.7K 閲覧数

16.5 : 緩衝液の有効性
酸塩基と溶解度平衡
48.5K 閲覧数

16.6 : 滴定計算:強酸-強塩基
酸塩基と溶解度平衡
29.0K 閲覧数

16.7 : 滴定計算:弱酸-弱塩基
酸塩基と溶解度平衡
43.8K 閲覧数

16.8 : 指示薬
酸塩基と溶解度平衡
47.8K 閲覧数

16.9 : 多価酸の滴定
酸塩基と溶解度平衡
95.7K 閲覧数

16.10 : 溶解平衡
酸塩基と溶解度平衡
52.0K 閲覧数

16.11 : 溶解性に影響する因子
酸塩基と溶解度平衡
33.0K 閲覧数

16.12 : 錯体イオンの形成
酸塩基と溶解度平衡
23.2K 閲覧数

16.13 : イオンの沈殿
酸塩基と溶解度平衡
27.6K 閲覧数

16.14 : 定性分析
酸塩基と溶解度平衡
21.5K 閲覧数
Copyright © 2023 MyJoVE Corporation. All rights reserved