4.4 : The Equilibrium Binding Constant and Binding Strength
The equilibrium binding constant (Kb) quantifies the strength of a protein-ligand interaction. Kb can be calculated as follows when the reaction is at equilibrium:
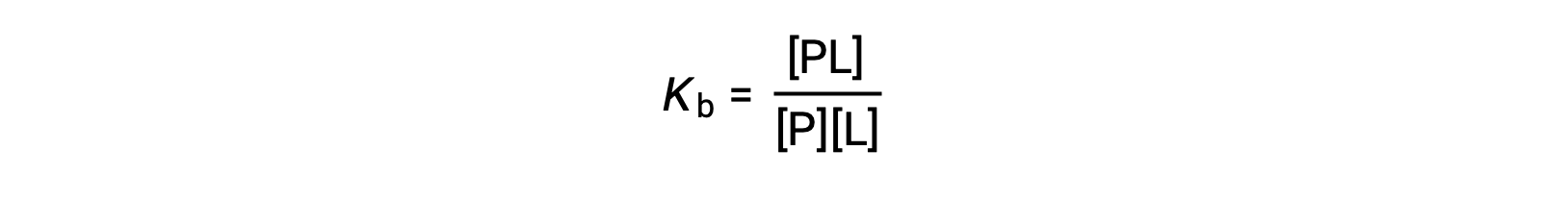
where P and L are the unbound protein and ligand, respectively, and PL is the protein-ligand complex.
As the amount of bound ligand is also related to the rate of ligand binding, experiments can also determine Kb by examining the rates of protein-ligand association (kon) and dissociation (koff) using the following ratio:
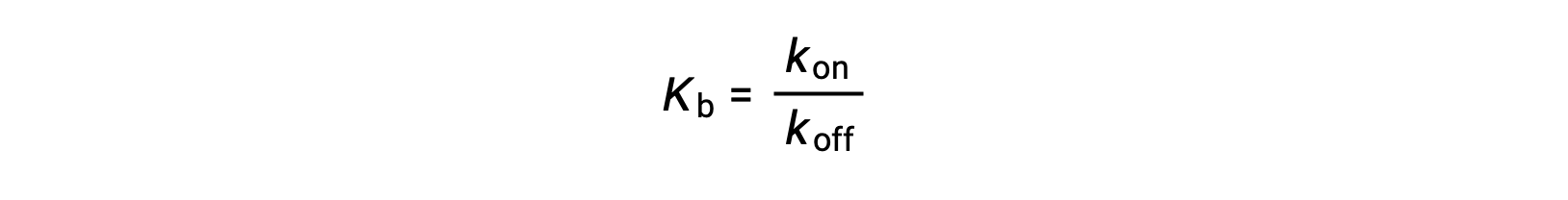
Thus, two categories of binding assays are used to determine the equilibrium binding constant – those that measure equilibrium concentrations and those that measure a reaction’s kinetics. In case, the reaction must be at equilibrium at the time of measurement.
The method of determining the equilibrium concentrations depends on the desired sensitivity and ease of signal detection. For these reasons, spectroscopic assays are most widely used. In these experiments, the reaction produces an absorbance change of a reactant or a product at a given wavelength, detectable by a UV-Vis spectrophotometer. Alternatively, the reactant or product can be tagged with a fluorescent probe or may contain an intrinsic fluorophore. Then, the reaction progress can be measured from the change in fluorescence. These assays are performed by varying one reactant’s concentrations while the rest of the experiment is held constant. The results can then be graphed and analyzed with various curve fitting methods.
Interactions between proteins and ligands are also studied using a variety of biochemical and spectroscopic techniques. Structural analysis, using X-ray crystallography and NMR spectroscopy, aids in predicting protein-ligand interactions through molecular simulations. Theoretical and computational approaches, such as protein-ligand docking studies, are used extensively to characterize the position and interactions of small molecule ligands, including drug candidates. Computer-aided drug design is a fast and low-cost alternative to accelerate the pace of conventional trial and error drug testing.
章から 4:

Now Playing
4.4 : The Equilibrium Binding Constant and Binding Strength
タンパク質の機能
12.7K 閲覧数

4.1 : リガンド結合部位
タンパク質の機能
12.6K 閲覧数

4.2 : タンパク質-タンパク質界面
タンパク質の機能
12.4K 閲覧数

4.3 : 保存された結合部位
タンパク質の機能
4.1K 閲覧数

4.5 : 補因子と補酵素
タンパク質の機能
7.2K 閲覧数

4.6 : アロステリックレギュレーション
タンパク質の機能
13.9K 閲覧数

4.7 : リガンド結合とリンケージ
タンパク質の機能
4.7K 閲覧数

4.8 : 協調的アロステリックトランジション
タンパク質の機能
7.8K 閲覧数

4.9 : リン酸化
タンパク質の機能
5.8K 閲覧数

4.10 : プロテインキナーゼとホスファターゼ
タンパク質の機能
12.9K 閲覧数

4.11 : GTPアーゼとその規制
タンパク質の機能
8.2K 閲覧数

4.12 : 共有結合タンパク質調節剤
タンパク質の機能
6.7K 閲覧数

4.13 : 交換可能な部品を持つタンパク質複合体
タンパク質の機能
2.5K 閲覧数

4.14 : 機械的タンパク質機能
タンパク質の機能
4.9K 閲覧数

4.15 : 構造タンパク質機能
タンパク質の機能
27.1K 閲覧数
See More
Copyright © 2023 MyJoVE Corporation. All rights reserved