20.18 : Maxwell's Thermodynamic Relations
Maxwell's thermodynamic relations are very useful in solving problems in thermodynamics. Each of Maxwell's relations relates a partial differential between quantities that can be hard to measure experimentally to a partial differential between quantities that can be easily measured. These relations are a set of equations derivable from the symmetry of the second derivatives and the thermodynamic potentials.
All thermodynamic potentials are exact differentials. Therefore, their second-order derivative does not depend on the order of differentiation. In the case of Maxwell's relations, the thermodynamic potential is expressed in terms of the partial derivatives of that function. Substituting the values of the partial derivatives gives Maxwell's equations. The four Maxwell's relations are:
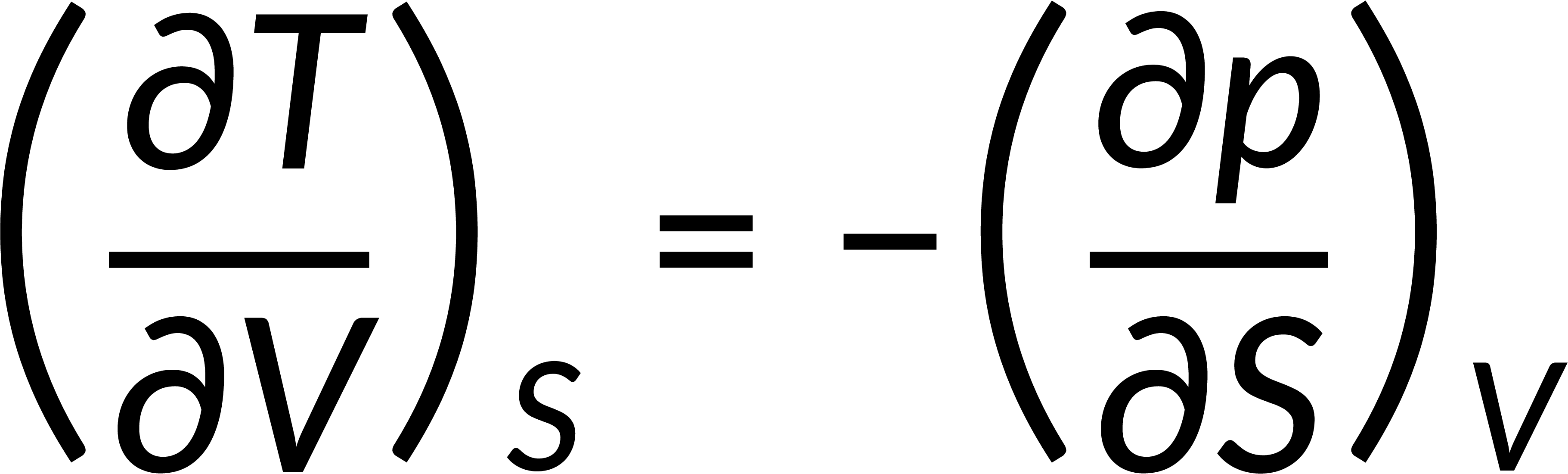
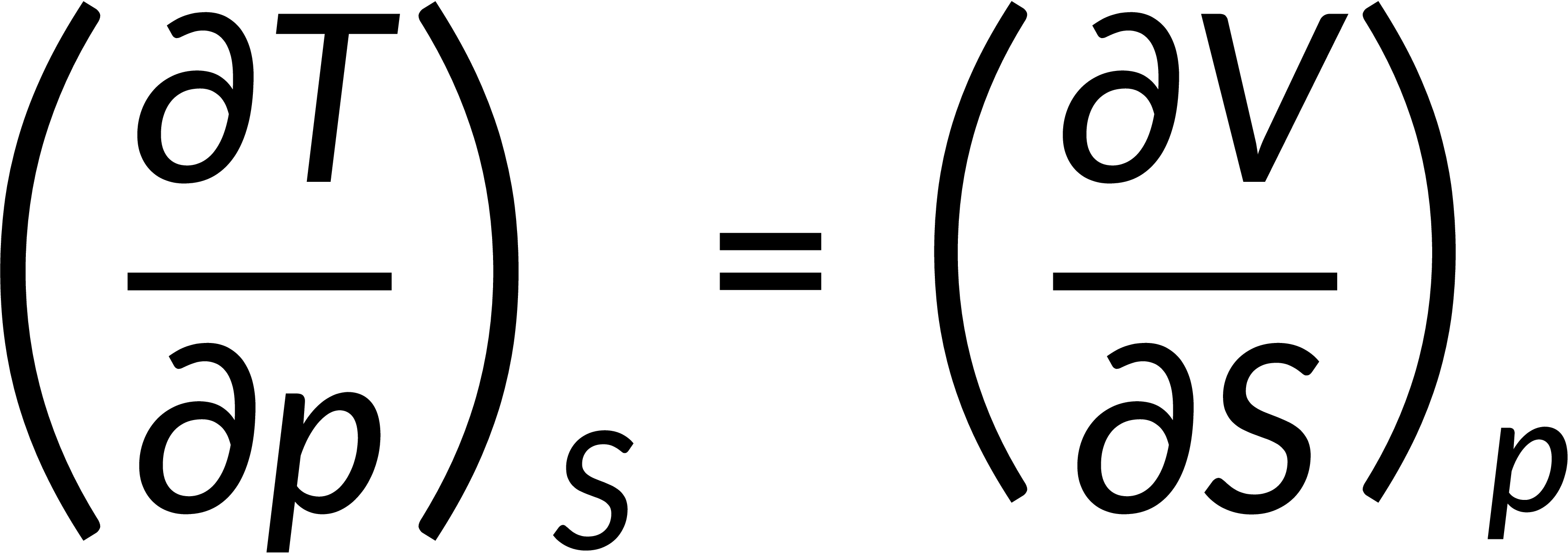
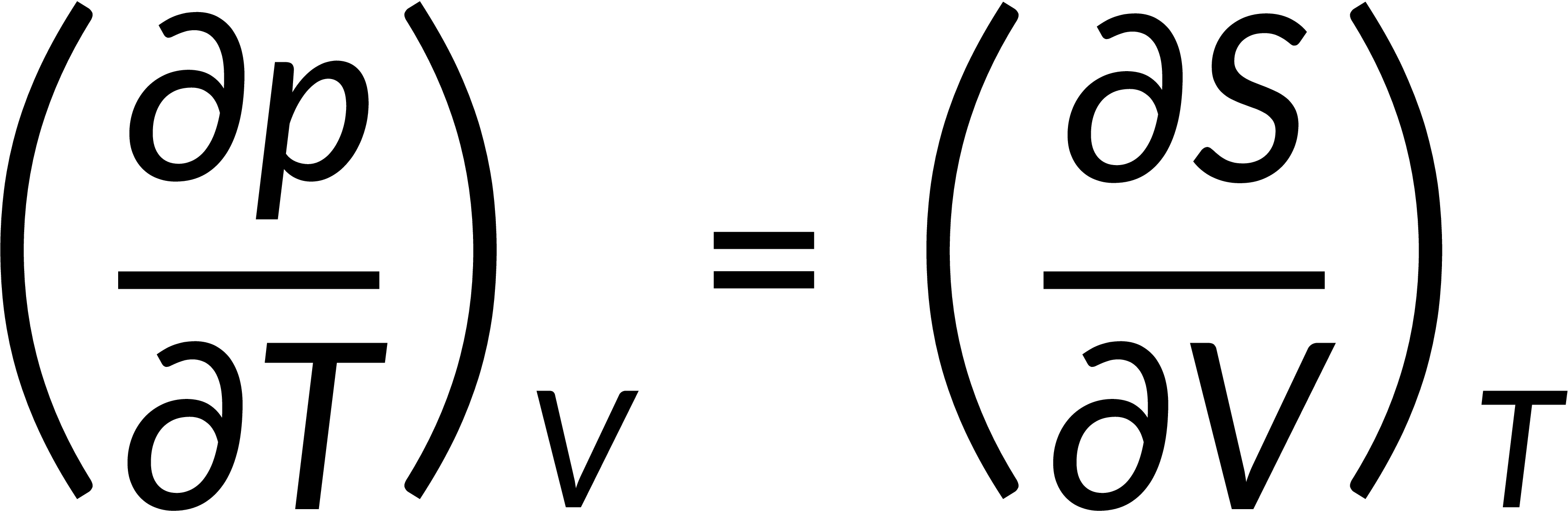
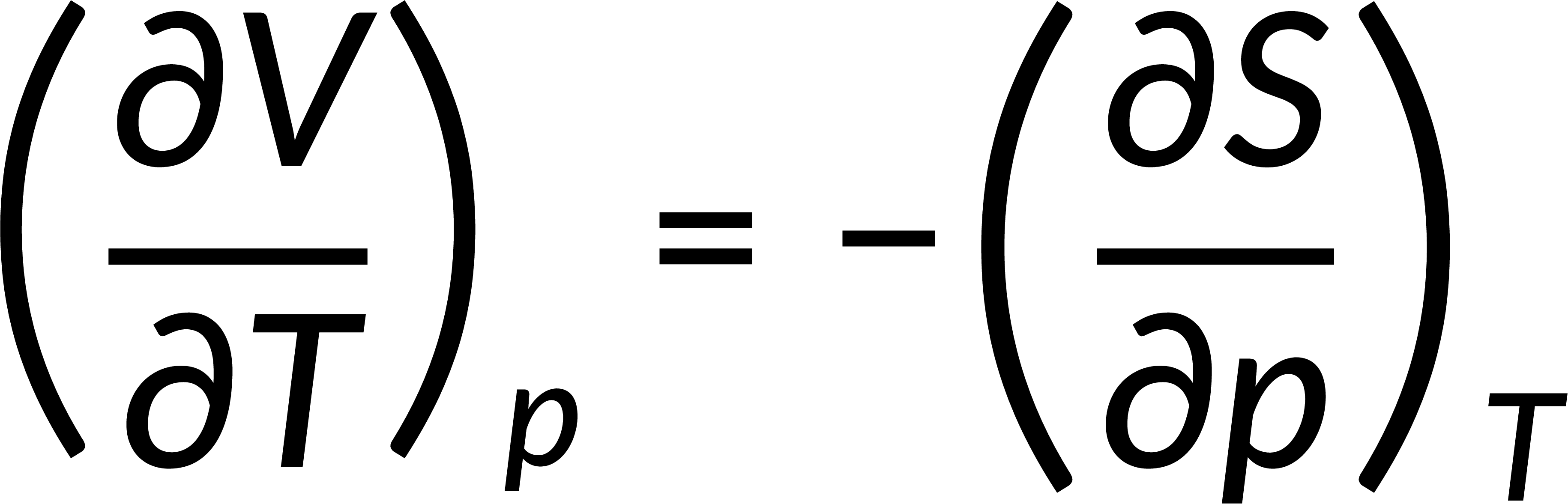
These equations relate entropy changes, which are difficult to measure, with changes in other thermodynamic variables, like temperature, volume, and pressure, that are easier to measure. For example, in the last equation, the left-hand side term gives the change in pressure with the temperature at constant volume. This quantity can be easily measured in a laboratory. However, the term on the right-hand side of the equation is more complicated, as it is hard to measure the entropy change with volume at a constant temperature.
章から 20:

Now Playing
20.18 : Maxwell's Thermodynamic Relations
熱力学の第一法則
2.4K 閲覧数

20.1 : 熱力学系
熱力学の第一法則
4.9K 閲覧数

20.2 : ボリューム変更中に行われる作業
熱力学の第一法則
3.8K 閲覧数

20.3 : 熱力学状態間のパス
熱力学の第一法則
3.0K 閲覧数

20.4 : 熱と自由膨張
熱力学の第一法則
1.7K 閲覧数

20.5 : 内部エネルギー
熱力学の第一法則
4.3K 閲覧数

20.6 : 熱力学の第一法則
熱力学の第一法則
4.0K 閲覧数

20.7 : 熱力学の第一法則:問題解決
熱力学の第一法則
2.4K 閲覧数

20.8 : サイクリックプロセスと孤立したシステム
熱力学の第一法則
2.7K 閲覧数

20.9 : 等温プロセス
熱力学の第一法則
3.5K 閲覧数

20.10 : アイソコリックプロセスとアイソバリックプロセス
熱力学の第一法則
3.3K 閲覧数

20.11 : 理想気体の熱容量I
熱力学の第一法則
2.5K 閲覧数

20.12 : 理想気体の熱容量II
熱力学の第一法則
2.3K 閲覧数

20.13 : 理想気体の熱容量III
熱力学の第一法則
2.1K 閲覧数

20.14 : 理想気体の断熱過程
熱力学の第一法則
3.0K 閲覧数
See More
Copyright © 2023 MyJoVE Corporation. All rights reserved