20.18 : Maxwell's Thermodynamic Relations
Maxwell's thermodynamic relations are very useful in solving problems in thermodynamics. Each of Maxwell's relations relates a partial differential between quantities that can be hard to measure experimentally to a partial differential between quantities that can be easily measured. These relations are a set of equations derivable from the symmetry of the second derivatives and the thermodynamic potentials.
All thermodynamic potentials are exact differentials. Therefore, their second-order derivative does not depend on the order of differentiation. In the case of Maxwell's relations, the thermodynamic potential is expressed in terms of the partial derivatives of that function. Substituting the values of the partial derivatives gives Maxwell's equations. The four Maxwell's relations are:
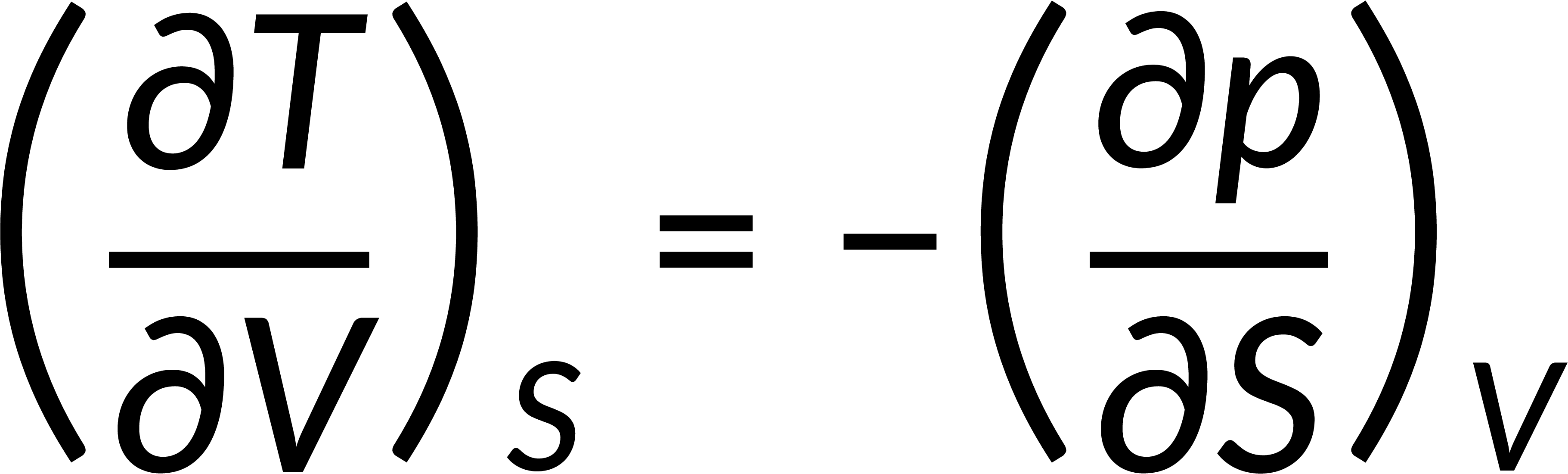
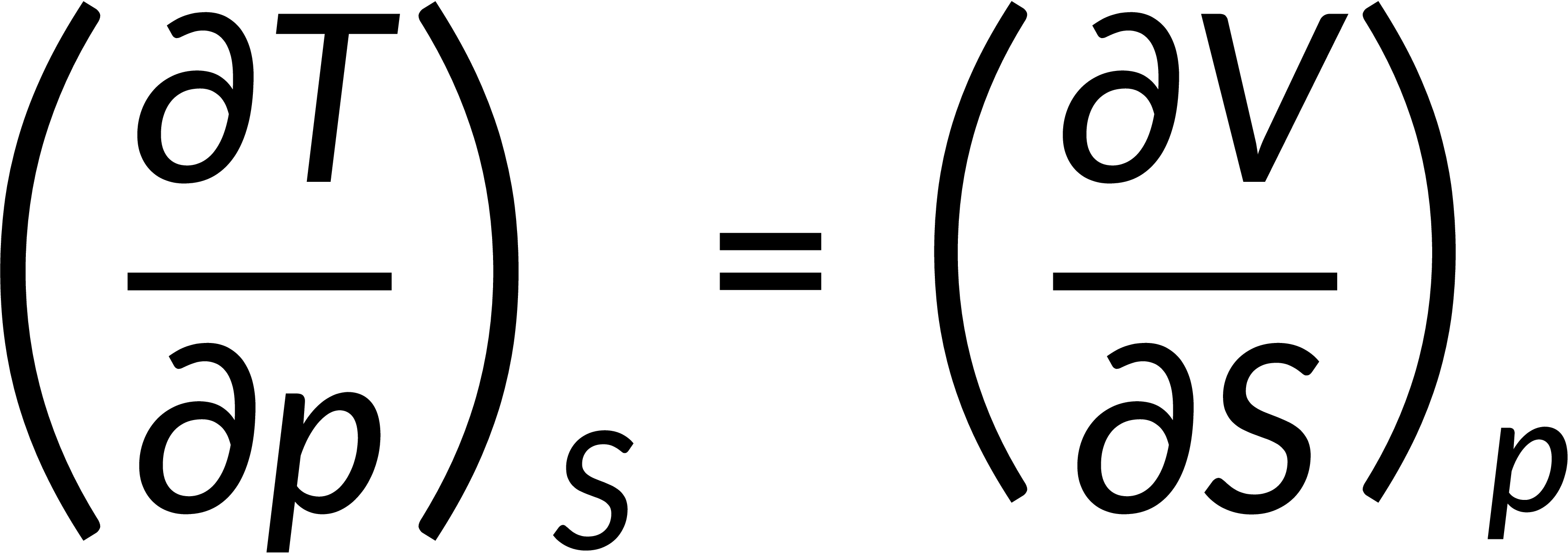
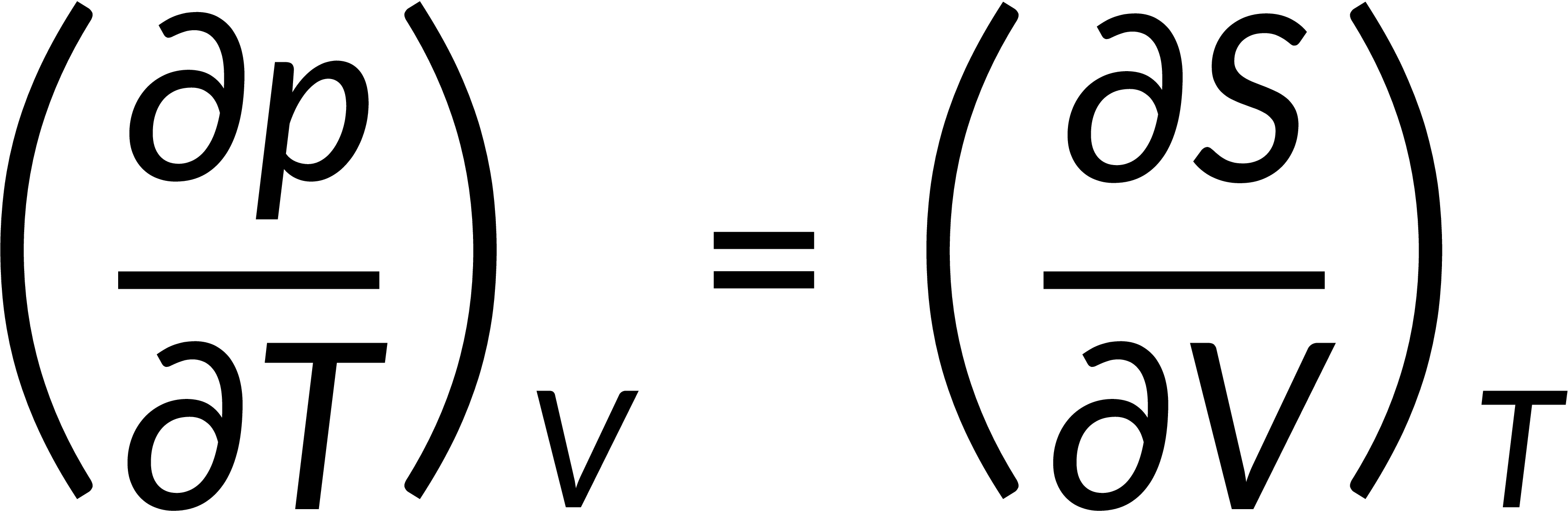
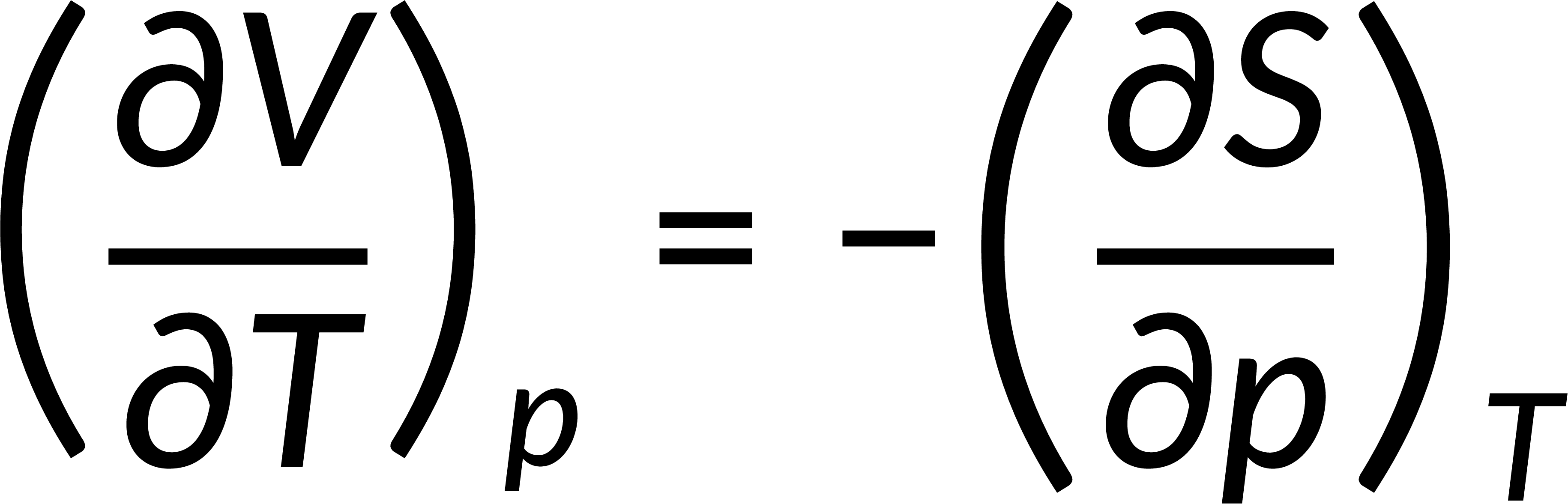
These equations relate entropy changes, which are difficult to measure, with changes in other thermodynamic variables, like temperature, volume, and pressure, that are easier to measure. For example, in the last equation, the left-hand side term gives the change in pressure with the temperature at constant volume. This quantity can be easily measured in a laboratory. However, the term on the right-hand side of the equation is more complicated, as it is hard to measure the entropy change with volume at a constant temperature.
Du chapitre 20:

Now Playing
20.18 : Maxwell's Thermodynamic Relations
The First Law of Thermodynamics
2.4K Vues

20.1 : Systèmes thermodynamiques
The First Law of Thermodynamics
4.9K Vues

20.2 : Travail effectué lors du changement de volume
The First Law of Thermodynamics
3.8K Vues

20.3 : Chemin entre les états thermodynamiques
The First Law of Thermodynamics
3.0K Vues

20.4 : Chaleur et détente libre
The First Law of Thermodynamics
1.7K Vues

20.5 : Énergie interne
The First Law of Thermodynamics
4.3K Vues

20.6 : Première loi de la thermodynamique
The First Law of Thermodynamics
4.0K Vues

20.7 : Première loi de la thermodynamique : résolution de problèmes
The First Law of Thermodynamics
2.4K Vues

20.8 : Processus cycliques et systèmes isolés
The First Law of Thermodynamics
2.7K Vues

20.9 : Procédés isothermes
The First Law of Thermodynamics
3.5K Vues

20.10 : Processus isochores et isobares
The First Law of Thermodynamics
3.3K Vues

20.11 : Capacités thermiques d’un gaz parfait I
The First Law of Thermodynamics
2.5K Vues

20.12 : Capacités thermiques d’un gaz parfait II
The First Law of Thermodynamics
2.3K Vues

20.13 : Capacités thermiques d’un gaz parfait III
The First Law of Thermodynamics
2.1K Vues

20.14 : Procédés adiabatiques pour un gaz parfait
The First Law of Thermodynamics
3.0K Vues
See More