20.18 : Maxwell's Thermodynamic Relations
Maxwell's thermodynamic relations are very useful in solving problems in thermodynamics. Each of Maxwell's relations relates a partial differential between quantities that can be hard to measure experimentally to a partial differential between quantities that can be easily measured. These relations are a set of equations derivable from the symmetry of the second derivatives and the thermodynamic potentials.
All thermodynamic potentials are exact differentials. Therefore, their second-order derivative does not depend on the order of differentiation. In the case of Maxwell's relations, the thermodynamic potential is expressed in terms of the partial derivatives of that function. Substituting the values of the partial derivatives gives Maxwell's equations. The four Maxwell's relations are:
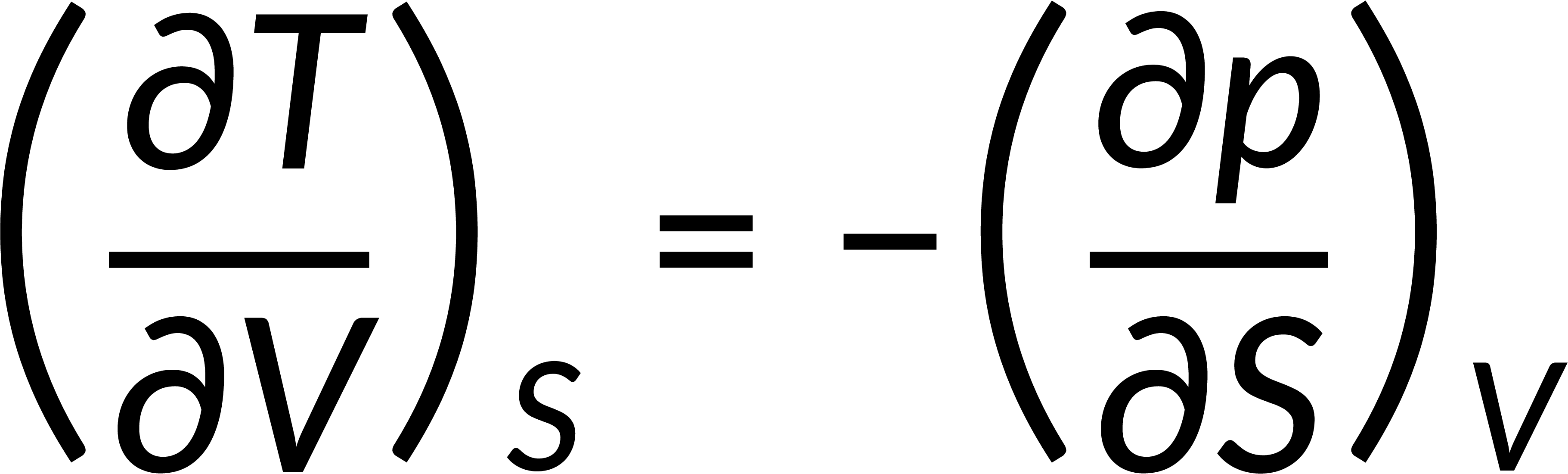
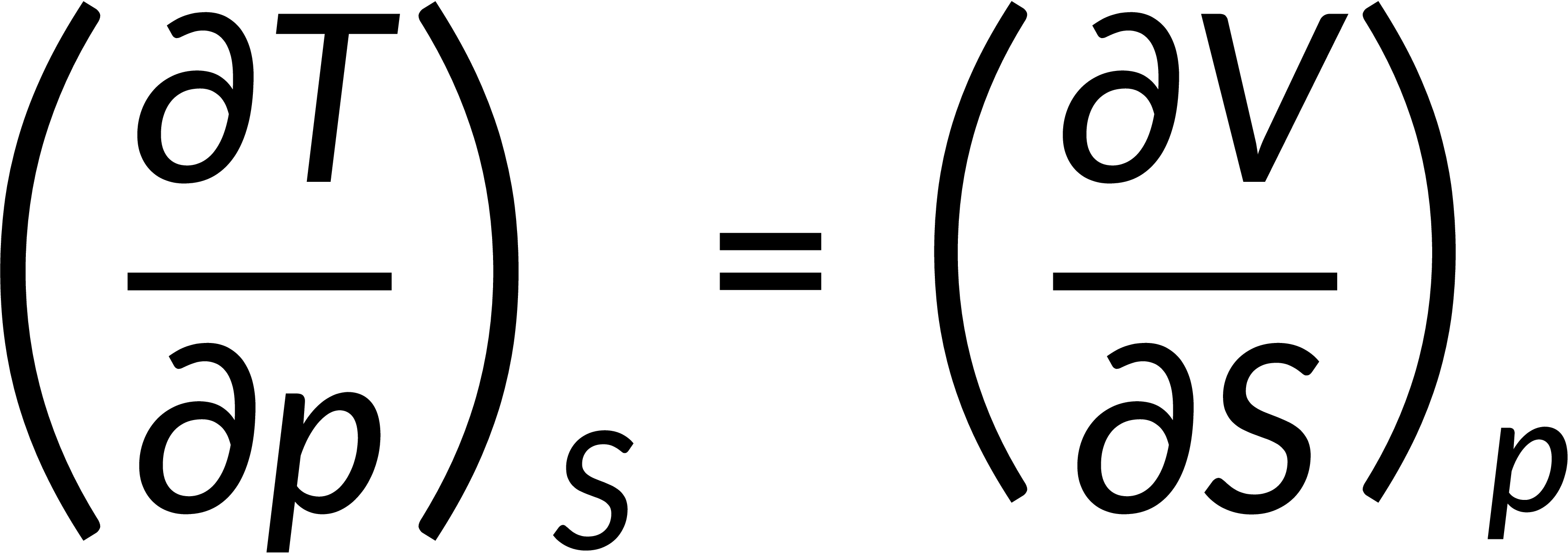
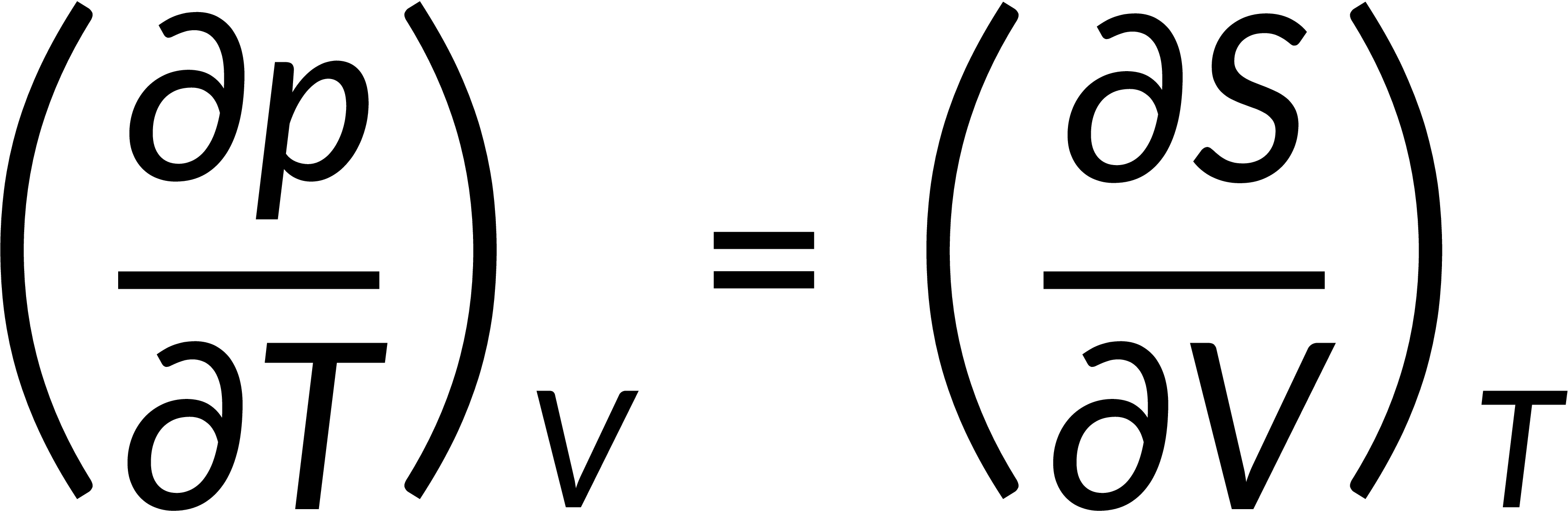
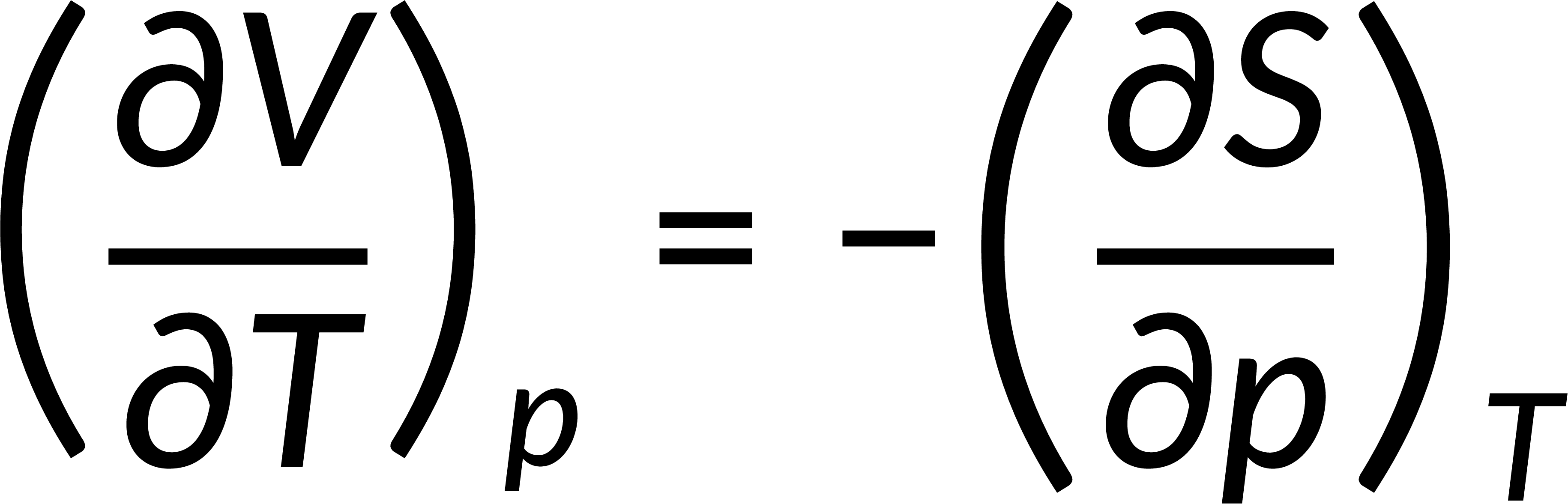
These equations relate entropy changes, which are difficult to measure, with changes in other thermodynamic variables, like temperature, volume, and pressure, that are easier to measure. For example, in the last equation, the left-hand side term gives the change in pressure with the temperature at constant volume. This quantity can be easily measured in a laboratory. However, the term on the right-hand side of the equation is more complicated, as it is hard to measure the entropy change with volume at a constant temperature.
Dal capitolo 20:

Now Playing
20.18 : Maxwell's Thermodynamic Relations
The First Law of Thermodynamics
2.4K Visualizzazioni

20.1 : Sistemi termodinamici
The First Law of Thermodynamics
4.9K Visualizzazioni

20.2 : Lavoro svolto durante la modifica del volume
The First Law of Thermodynamics
3.8K Visualizzazioni

20.3 : Percorso tra gli stati della termodinamica
The First Law of Thermodynamics
3.0K Visualizzazioni

20.4 : Calore ed espansione libera
The First Law of Thermodynamics
1.7K Visualizzazioni

20.5 : Energia interna
The First Law of Thermodynamics
4.3K Visualizzazioni

20.6 : Primo principio della termodinamica
The First Law of Thermodynamics
4.0K Visualizzazioni

20.7 : Primo principio della termodinamica: risoluzione dei problemi
The First Law of Thermodynamics
2.4K Visualizzazioni

20.8 : Processi ciclici e sistemi isolati
The First Law of Thermodynamics
2.7K Visualizzazioni

20.9 : Processi isotermici
The First Law of Thermodynamics
3.5K Visualizzazioni

20.10 : Processi isocorici e isobarici
The First Law of Thermodynamics
3.3K Visualizzazioni

20.11 : Capacità termiche di un gas ideale I
The First Law of Thermodynamics
2.5K Visualizzazioni

20.12 : Capacità termiche di un gas ideale II
The First Law of Thermodynamics
2.3K Visualizzazioni

20.13 : Capacità termiche di un gas ideale III
The First Law of Thermodynamics
2.1K Visualizzazioni

20.14 : Processi adiabatici per un gas ideale
The First Law of Thermodynamics
3.0K Visualizzazioni
See More