JoVE 비디오를 활용하시려면 도서관을 통한 기관 구독이 필요합니다. 전체 비디오를 보시려면 로그인하거나 무료 트라이얼을 시작하세요.
Viral-Mediated Labeling and Transplantation of Medial Ganglionic Eminence Cells for In Vivo Studies
Overview
This video demonstrates the viral labeling and transplantation of medial ganglionic eminence (MGE) cells for in vivo studies. It outlines the steps involved in transducing MGE cells with a viral reporter, transplanting these transduced cells into the cortex of a mouse pup, and enabling reporter expression in specific interneuron subtypes.
프로토콜
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Lentivirus Preparation (Optional Step)
- Split three 10 cm plates of HEK293T cells for each lentivirus to be made in the presence of 10 ml DMEM/10% FBS and grow to ~60-70% confluence. Grow cells in an incubator at 37 °C with 5% CO2.
- Transfect the following DNA plasmids (10 µg total) into each plate using any transfection reagent: 6.4 µg CAG-Flex-GFP lentiviral vector, 1.2 µg pMD2.G (encodes VSV-g), 1.2 µg pRSV-Rev, and 1.2 µg pMDLg/pRRE. The three lentiviral packaging vectors are commercially available.
Note: This approach utilizes 3rd generation lentiviral vectors, but 2nd generation vectors and associated plasmids will also work. - Prepare a waste container containing a 10% bleach solution within a BSL2 certified hood to inactivate any solutions or devices that contacted or contain lentivirus. Next, completely remove the media 4-6 hr after transfection into the bleach solution, replace with the same volume of new media, and allow the cells to grow.
- After 4 days, collect media into 50 ml conical tubes and centrifuge at 1,000 x g for 15 min to pellet any cell debris. Next, filter the supernatant through a 0.45 µm membrane. Any low protein binding filter, like PVDF, works well. Caution: Place both solid and liquid viral waste into the bleach solution.
- Load 30 ml filtered supernatant into ultracentrifuge tubes and into an ultracentrifuge. Centrifuge at 100,000 x g for 2.5 hr at 4 °C. Open the centrifuge tubes in a BSL2 hood and remove supernatant into 10% Bleach. Add ~100 µl of PBS to the pellet, which yields titers of 1x107 to 1x108 infectious units/ml. Aliquot the following day and store at -80 ºC. Lentivirus aliquots are stable at -80 °C for at least six months.
- Important consideration: Many institutions now have viral cores. Acquire concentrated virus with a minimum titer of 1x107 infectious units/ml, for optimal results.
2. Donor Mice for MGE Dissection
- First, set up a timed mating between a Cre-expressing line of interest and either a wild type (WT) mouse or a mouse that will express a reporter after Cre-mediated recombination.
Note: Many Cre-driver lines are transgenic and are maintained and bred in the hemizygous state. Thus, only half of the embryos will contain the Cre transgene. DNA can be quickly prepared from the embryos for a short PCR to detect the Cre allele. The Cre+ embryos can then be harvested so that only the MGE tissue of the desired genotype is used. Alternatively, a Cre-dependent reporter may be used to select the embryos for MGE dissection and transplantation. - Calculate which day will correspond to E13.5. If animals were paired the evening before, the day of the plug is 0.5. Order timed-pregnant mice or a WT female with P1 pups in order to time having postnatal (P)1 mice on the day the donor tissue will be E13.5. Alternatively, set up these matings in house one week prior to pairing the donor animals.
Note: The former strategy of generating both E13.5 and P1 embryos/pups for the same day is often difficult to do reliably.
3. Preparation of Media, Tools and Equipment.
- Prepare the reagents and tools for MGE cell preparation (Table 1).
- Prepare a clean surface and place clean forceps, scissors and either a microsurgical knife or fine forceps (for precise tissue dissection) on the surface.
- Prepare Hank's balanced salt solution (HBSS) for dissection and Dulbeco's modified eagle's medium (DMEM) with 10% fetal bovine serum (FBS) for transduction.
- Preincubate 20-30 ml DMEM/10% FBS in a 37 °C tissue culture incubator for about 1 hr. The lid should be loosened to allow for gas exchange and pH equilibration of the media.
Note: The media preincubation can be prepared just before starting the dissection.
4. MGE Cell Preparation
- Sacrifice the pregnant mouse according to an approved animal protocol and remove the embryos into a 10 cm petri dish containing ice-cold HBSS.
- Remove the brain from each embryo using a dissecting scope (do one at a time, leaving the rest of the embryos in ice-cold HBSS) and then proceed to cut out the MGE tissue.
Note: Detailed below and in Figure 1 are key steps showing how to remove the MGE.- Position the brain with the ventral side facing up (Figures 1A, A'). Note: it is also okay to have the dorsal aspect facing up. Cut the brain in half along the sagittal plane to generate two pieces. An example hemisected brain is shown (Figures 1B, B'); notice that dorsal (the overlying cortex) and ventral aspects of the brain cover and surround the ganglionic eminence (GE) tissue.
- Next, with a dominant hand, hold either very fine forceps or a stab knife (Table 1) while immobilizing the hemisphere with forceps held by a non-dominant hand. (The hemisphere's medial aspect should be facing up). Unfold the dorsal and ventral tissue with forceps and a stab knife to reveal the underlying GE tissue (Figure 1C and diagrammed in Figure 1C').
Note: Removing some media from the dissection petri dish can make it easier to stabilize the tissue while performing the MGE dissection. - Make straight cuts with the stab knife or fine forceps to separate the CGE, LGE, and septal tissues (see example cuts denoted by red dashed lines, Figures 1D and D'). These cuts can be made in any order.
Note: Imagine the MGE as a 'box' that is cut out of the surrounding tissue. Doing so helps reproducibly make similar cuts for each dissection, thus reducing variability in tissue dissections. - Finally, turn the MGE on its side and trim off the bottom (what was the lateral aspect of the brain). This removes the mantle zone of the MGE (discard in Figures 1E and 1E') from the rest of the MGE. The remaining aspect of MGE primarily contains ventricular zone and subventricular zone portions of the MGE, which contain nearly all the MGE progenitor cells. Proceed with this tissue.
- Important consideration: The addition of silicone gel to the bottom of a petri dish can serve as an excellent dissection surface as it protects the delicate tips of forceps and provides a soft surface for pinning tissue with forceps.
5. Lentiviral Labeling and Transplantation
- Move the tubes of MGE tissue into a BSL2 certified hood and remove the media.
Note: The MGE tissue is big enough to be visible and will settle to the bottom of the tube, allowing the cold media to be easily and gently removed. - Add ~500 µl of DMEM/10% FBS media, that was preincubated in a 37 °C tissue culture incubator. Next, add polybrene to facilitate transduction to each tube at a final concentration of 8 µg/ml. Triturate the MGE tissue to create a single cell suspension using a P1000 pipette tip. Finally, add ~15-20 µl of concentrated lentivirus to each tube.
- Important consideration: A trituration of 10-12 times is sufficient to break up MGE tissue from a single embryo, but more triturations may be required if multiple MGEs are pooled together. Try out different trituration conditions to determine what works best.
- Securely close each tube, invert to mix, then place all the tubes in a 37 °C incubator. Incubate the cells in lentivirus for at least 30 min and up to 1 hr, longer times have resulted in decreased cell viability. Invert the tubes every 10 min until the time is up.
- After incubation with lentivirus, remove the tubes from the incubator and centrifuge at ~700 x g for 3 min to pellet the cells. In the BSL2 hood, remove the supernatant and discard into 10% bleach. Next, add 1 ml DMEM/10% FBS and triturate the pellet 2-3 times to wash, then centrifuge again at the same speed to pellet the cells. Repeat this wash step 2-3 more times to remove excess virus.
Note: Perform centrifugation steps at 4 °C; however, reduced viability has not been observed when short spins are performed at RT. - After the final wash, remove as much media as possible and put each tube on ice. Due to residual media on the sides of the tube, ~2-3 µl of media will end up covering the cell pellet by the time the transplantation procedure has begun.
6. Transplantation and Validation
- Acquire host P1 pups and prepare the procedure area by spraying down with 70% ethanol. To maintain sterility during the procedure it is recommended to perform these procedures in a dedicated clean procedure room. In addition, use autoclaved surgical tools and sterilize the surfaces the pups will be in contact with, using 70% ethanol. An example of representative equipment needed to perform the transplantations, and a representative procedure area, is shown in Figure 2.
Note: While this procedure is an injection of small volumes, and not a surgical procedure that would require an incision, open wound or sutures, it is still recommended that a new micropipette is used for each mouse to be injected. The micropipettes are heat sterilized when pulled and beveled on a surface that was sprayed with 70% ethanol being stored in a sealed container.- Generate micropipettes to have a diameter at the tip between 40-80 µm. Following these dimensions will yield optimal results. Heat-sterilize the micropipettes when pulled and beveled on a surface and spray with 70% ethanol before storing the micropipettes in a sealed container.
Note: Alternatively, if access to the devices needed to create these needles (Table 1) is limiting, use any other injection device. However, these may not be as well suited as the micropipettes due to different diameters. - Draw up mineral oil into a 1 ml syringe, and with a 30 1/2 G needle, fill the glass pipette completely with mineral oil. Next, mount the plunger onto the stereotaxic device, then attach the glass pipette to the stereotaxic device and load it onto the plunger. Using the hydraulic drive, move the plunger about halfway into the glass pipette, removing mineral oil that spills out with a clean paper towel.
- Alternative to using a syringe, submerge the back end of the pipette in mineral oil and fill by capillary action. To prevent air bubbles in the pipette, maintain positive pressure on the syringe until completely out of the glass pipette.
Note: Other devices can be used to successfully transplant MGE cells. Any device that can deliver a volume less than or near 100 nl works well for this procedure.
- Alternative to using a syringe, submerge the back end of the pipette in mineral oil and fill by capillary action. To prevent air bubbles in the pipette, maintain positive pressure on the syringe until completely out of the glass pipette.
- Next, use a sterile paper towel or any absorbent material, with the corner twisted into a fine point to delicately remove excess media above the MGE cell pellet. This step concentrates the cell density so that smaller injection volumes can be achieved. Next, use a low volume (P2 is preferred) pipette to slowly draw up the cell pellet and triturate 1-2 times before moving the cell suspension onto a hydrophobic surface. The volume should be just under 1 µl. Move the tip of the needle into the cell suspension and using the hydraulic drive, draw up the cell suspension into the pipette.
- Anesthetize a P1 pup via hypothermia (wrap in a latex glove and put on ice for ~ 2-4 min). Check for effectiveness of anesthesia with a noxious stimulus. Pinch the skin between the toes with fine forceps: the pup should be unresponsive if pinched. Next, place the pup onto a mold that is under the injection device, then make the skin taut by pulling back the skin on the head and securing with standard lab tape.
Note: While hypothermia is very effective on neonatal mice and results in a low mortality rate, procedures must be done quickly, as pups will begin to recover in under 10 min. In addition, 4 min on ice is an upper limit of what can be tolerated. Try to only induce hypothermia once if a longer time is required for injections. However, if a pup recovers early, first allow the pup to fully recover before inducing hypothermia again. Moreover, only induce hypothermia one more time to perform injections. Pups will recover within 5-10 min of hypothermia. This can be facilitated by placing the pups with litter and mom or by placing the pup on a warm surface to recover. - Using a stereotaxic device, position the tip of the glass pipette on the surface on the pup's head, perpendicular to the surface of the head. When the pipette is in contact with the head, consider this as '0'. Next, push the pipette through the surface of the skin and skull into the cortex, then insert and retract the pipette ~ 2 times before stopping. For optimal targeting of cells into the neocortex, injections are performed when the micropipette is at a depth of 0.1 mm. However, this should be optimized by the user (see note below).
Note: While this depth reliably targets deeper neocortical layers with the devices described above, a few depths should be tested and determined empirically. In addition, a range of depths at different rostro-caudal and medio-lateral position should be tested to determine what depth is optimal at each site. Also, the initial puncture should be rapid, as slow penetration into the neocortex diminishes the ability to cleanly penetrate the tissue. Try different speeds when first starting this procedure to find what works best. In addition, for testing coordinates, there is no real substitute for using labeled cells, as dyes have been unreliable to denote position. - Once the needle is in position, rotate the hydraulic device to advance the plunger into the glass pipette, pushing a set volume of cell suspension into the cortex. Inject 50-70 nls per site. Repeat this procedure at 3-6 different sites at different rostral-caudal levels if the goal is to obtain a wide distribution of cells throughout the neocortex.
Note: Volumes of 100 nl or greater can cause lesions and lead to large clumps of injected cells that fail to efficiently migrate out of the injection site. Thus, smaller injection volumes (~70 nl or less) are optimal, over more sites if time permits. - When injections are complete, remove the pup from the stage and mark it (toe clipping is a reliable way to identify the pups later). Once the pup has recovered and is able to move on its own (this occurs within minutes of removing them from the stage), put it back with the mom and litter.
Note: Since this is a minimally invasive procedure, there are no post-surgical procedures once the pup is freely moving and warmed. However, pups should be checked not only after the procedure but also the following day to assure they have no signs of deteriorating health. - Before starting the procedure on the next pup, spray down the area with 70% ethanol to maintain a sterile working area. Allow the injected pups to develop and then assess the tissue at the appropriate developmental stage(s).
- Generate micropipettes to have a diameter at the tip between 40-80 µm. Following these dimensions will yield optimal results. Heat-sterilize the micropipettes when pulled and beveled on a surface and spray with 70% ethanol before storing the micropipettes in a sealed container.
Table 1. Inventory list of reagents and tools. An inventory of commercially available reagents including media, dissection tools, representative equipment for transplantation and DNA vectors. (n.a.) Not available or applicable.
| Reagents and equipment for MGE cell preparation and transduction | Catalog # | Company | ||
| 1) | Dulbecco's Modified Eagle Medium, with high glucose | 12491-015 | Life Technologies | |
| 2) | Heat inactivated Fetal Bovine Serum | 10437-077 | Life Technologies | |
| 3) | Hanks balanced salt solution (HBSS), no calcium or magnesium | 14170-112 | Life Technologies | |
| 4) | 1.5 ml microcentrifuge tubes (or other collection tubes with lids) | 3810X | Eppendorf International | |
| 5) | Polybrene | sc-134220 | SantaCruz Biotechnology | |
| 6) | Stab knife straight 22.5 ° (optional) | REF 72-2201 | Surgical specialities corporation | |
| 7) | Petri dish (10 cm), for tissue dissections | FB0875713 | Fisher Scientific | |
| 8) | 2 fine tip forceps, like Dumont #5 | 11254-20 | Fine Scientific Tools | |
| 9) | 37 °C tissue culture incubator with 5% CO2 input | C150 | Binder | |
| 10) | Tabletop centrifuge that can spin 1.5 ml microcentrifuge tubes | |||
| 11) | Any P1000 pipette that can be used for cell trituration | |||
| 12) | Biosafety level 2 hood | |||
| Reagents and equipment for in vitro MGE primary cultures | Catalog # | Company | ||
| 1) | Lab-TekII chamber slides with cover, 8 well | 154941 | Thermo Fisher | |
| 2) | Poly-L-lysine 0.1% weight/volume | P8920 | Sigma Aldrich | |
| 3) | Mouse Laminin, 0.5-2 mg/ml | 23017-015 | Life Technologies | |
| 4) | Neurobasal medium | 21103-049 | Life Technologies | |
| 5) | B27 serum free supplement | 17504044 | Life Technologies | |
| 6) | Glutamax, 100x stock | 35050-061 | Life Technologies | |
| 7) | Penicillin-Streptomycin, 100x stock | 15070-063 | Life Technologies | |
| 8) | Glucose, (prepare a 25% solution in water) | G5400 | Sigma Aldrich | |
| Reagents and equipment for MGE cell transplantation | Catalog # | Company | ||
| 1) | 1 ml syringe | REF 309602 | BD, Becton Dickinson and company | |
| 2) | 30 1/2 G needle | 305106 | BD, Becton Dickinson and company | |
| 3) | Precision bore to deliver 5 µl (comes with plunger) | 5-000-1005 | Drummond scientific company | |
| 4) | Parafilm | PM-999 | Polysciences, Inc. | |
| 5) ** | Stereo microscope with boomstand | MZ6 | Leica | |
| 6) ** | Digital just for mice stereotaxic instrument | 51725D | Stoelting company | |
| 7) ** | Single-axis oil hydraulic fine micromanipulator | MO-10 | Narishige | |
| 8) | Diamond coated rotary beveler | n.a. | made in house | |
| 9) | Needle pipette puller | 730 | Kopf instruments | |
| 10) | Mineral oil | n.a. | Any drug store or pharmacy | |
| 11) | Any pipette and tips that can reliably measure 1 µl of volume | |||
| ** These are the models we use, but many other setups should work | ||||
| Optional Reagents (for making Lentiviruses) | Catalog # | Company | ||
| 1) | 25 mm, 0.45 µm filter | 09-719B | Fisher Scientific | |
| 2) | Ultra-clear centrifuge tubes (25 x 89 mm) | 344058 | Beckman Coulter® | |
| 3) | Lipofectamine 2000 | (1.5 ml size) | 11668019 | Invitrogen |
| Other commercially available supplies | Catalog # | Company | ||
| 1) | pMDLg/pRRE plasmid encoding gag/pol | 12251 | Addgene | |
| 2) | pRSV-Rev plasmid encoding Rev | 12253 | Addgene | |
| 3) | pMD2.G plasmid encoding VSVG | 12259 | Addgene | |
| 4) | pAAV-Flex-GFP | 28304 | Addgene | |
결과
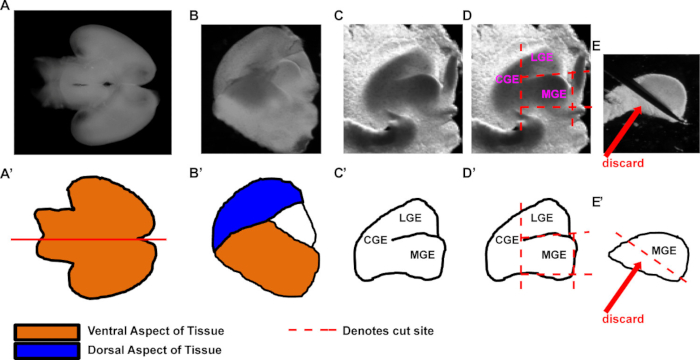
Figure 1. Representative procedure for the dissection of E13.5 MGE tissue. Example dissection of MGE tissue for either primary cultures or transplantations. (A-E) Images of E13.5 brain dissections, and (A'-E') schematics to highlight structures and important steps in the procedure. (A, A') Repre...
공개
자료
| Name | Company | Catalog Number | Comments |
| Reagents and equipment for MGE cell transplantation | |||
| 1 ml syringe | REF 309602 | BD, Becton Dickinson and company | |
| 301/2 gauge needle | 305106 | BD, Becton Dickinson and company | |
| Precision bore to deliver 5 µl (comes with plunger) | 5-000-1005 | Drummond scientific company | |
| Parafilm | PM-999 | Polysciences, Inc. | |
| Stereo microscope with boomstand ** | MZ6 | Leica | |
| Digital just for mice stereotaxic instrument ** | 51725D | Stoelting company | |
| Single-axis oil hydraulic fine micromanipulator ** | MO-10 | Narishige | |
| Diamond coated rotary beveler | n.a. | made in house | |
| Needle pipette puller | 730 | Kopf instruments | |
| Mineral oil | n.a. | Any drug store or pharmacy | |
| Any pipette and tips that can reliaby measure 1 µl of volume | |||
| ** These are the particular models we use, but many other setups should work | |||
| Optional Reagents (for making Lentiviruses) | |||
| 25 mm, 0.45 µm filter | 09-719B | Fisher Scientific | |
| Ultra-clear centrifuge tubes (25 x 89 mm) | 344058 | Beckman Coulter® | |
| Lipofectamine 2000 (1.5 ml size) | 11668019 | Invitrogen | |
| Other commercially available supplies | |||
| pMDLg/pRRE plasmid encoding gag/pol | 12251 | Addgene | |
| pRSV-Rev plasmid encoding Rev | 12253 | Addgene | |
| pMD2.G plasmid encoding VSVG | 12259 | Addgene | |
| pAAV-Flex-GFP | 28304 | Addgene |
This article has been published
Video Coming Soon
Source: Vogt, D. et al., Viral-mediated Labeling and Transplantation of Medial Ganglionic Eminence (MGE) Cells for In Vivo Studies. J. Vis. Exp. (2015).
Copyright © 2025 MyJoVE Corporation. 판권 소유