Method Article
Detection of Vascular Pathways of Oral Mucosa Influencing Soft- and Hard Tissue Surgeries by Latex Milk Injection
In This Article
Summary
Here, we describe the staining of blood vessels using latex milk injections. This procedure provides anatomical knowledge of the oral blood supply for detailed macroscopic vascular mapping of the oral mucosa, as well as understanding of proper flap design to prevent complications and promote postoperative wound healing.
Abstract
In this methodological study, the purpose was to visualize the macroscopic vascular pathway of the oral mucosa. Corpses were injected and fixed with Thiel's solution for embalming to keep the natural color, fresh texture, and elasticity of the tissues. Latex milk injection is a technique used to stain blood vessels. A combination of Thiel’s embalming method and latex milk injection allows surgeons to work on a raw specimen and identify the distribution and anastomosis of vessels macroscopically in the oral mucosa for flap/incision design in periodontal and maxillofacial surgeries. The latex milk contains radiopaque material that enables clinicians to visualize the pathway of the blood vessels radiographically. A step-by-step protocol is described for the correct adjustment of Thiel embalming and latex milk injection. The combined application of both methods allows the clinician to understand anatomical structures practically. As a result, proper incisions and flaps can be designed, which prevents neurovascular damage, intraoperative bleeding, and postoperative morbidity during patient surgery.
Introduction
In order to design an appropriate incision or flap in implant, dentoalveolar, and periodontal surgeries, anatomical knowledge is crucial1,2. The oral mucosa possesses an intricate pattern of blood vessels arising from the subbranches of the external carotid artery (ECA). The maxilla and the mandible together with their surrounding connective tissues are mainly perfused by the branches of the maxillary artery (MA), the facial artery (FA), and the lingual artery (LA)3,4,5. From a clinical point of view, the visualization of the blood vessel pathways is necessary for clinicians to decrease intraoperative bleeding and postoperative morbidity6. Clinicians performing oral surgical interventions must be aware of the location of the vascular pathways to establish optimal conditions for wound healing and angiogenesis7. A detailed description of the arterial and venous distribution of the oral mucosa would enable oral surgeons and periodontists to minimize the risk of complications during surgery by optimized planning.
There are various methods in the literature to stain the blood vessels of the oral mucosa, such as India ink injection and corrosion casting8,9,10. One of the preferred staining methods uses latex milk7,8,9,10,11,12. As Haenssgen12 defined it, latex milk as a liquid complex emulsion formed by tannins, proteins, resins, sugars, oils, alkaloids, and gums that coagulates when exposed to air12. The latex is generally mixed with a red color agent and injected into the common carotid artery (CCA) or ECA to visualize the course of their branches and subbranches. The blood vessels are stained red and become more prominent during dissection. This method provides valuable data for clinicians through the visualization of blood vessels by definitive ex vivo macro- and microscopic analysis establishing a solid anatomical basis. Due to the presence of a radiopaque substance in the latex milk, the method displays excellent visualization in both traditional X-rays and 3D computed tomography (CT)5.
Several types of fixative solutions that can be used for embalming head specimens13,14,15 are explained and compared in the literature. Walter Thiel introduced Thiel’s solution in 199216,17. This solution conserves the natural color of tissues and vessels16,17,18,19,20. It has no detectable odor and maintains tissue elasticity of body parts as well as antimicrobial preservation of cadavers, and it can be useful for the development of new surgical devices or evaluation of a surgeon’s skill16,17,18,19,20.
Protocol
The method of latex milk injection was used bilaterally on human cadavers, which were donated to the Department of Macroscopic and Clinical Anatomy of the Medical University of Graz, Austria, complying with the Department’s Donation Program and according to Styrian burial law. 10 fresh cadavers (5 male-5 female, age 43–90) were selected. The path of the blood vessels in oral mucosa, utilized in combination with a layer-by-layer dissection protocol and CT, were analyzed.
Latex milk injection together with the examination of the blood vessels in the oral mucosa (Figure 1 and Figure 2)
- Penetrate the center of the sagittal suture between the parietal bones with a drill (see Table of Materials) to reach into the superior sagittal sinus.
- Design an incision in the carotid triangles parallel to the sternocleidomastoid muscle by Nr. 20 or Nr. 15 surgical blades. Then, separate the edges of the skin, adipose tissue, and the muscles with a Weitlaner retractor (140 mm, 3/4 teeth) to expose the surgical site.
- Gently reveal the CCAs using Nr. 15 surgical blades and block the end using serrated dressing forceps.
- After that, cannulate the CCAs and superior sagittal sinus carefully with a metal catheter.
- Isolate the catheter using a ligature with alternating knots on each side of the CCA. Fix the catheter very securely to prevent any leakage of Thiel's intravascular solution or latex milk when injected under pressure.
- Subsequently, perfuse the corpse tissues via the CCAs and superior sagittal sinus simultaneously with an intravascular solution (e.g., Thiel's or phosphate buffer solution) using an air pressure pump system (0.25–0.5 bar) for ~10–15 min.
NOTE: The main components of the intravascular and embalming Thiel's solution are composed of ammonium nitrate (fixative), potassium nitrate (fixative), 4-chloro-3-methylphenol (fixative), sodium sulfite (fixative), boric acid (disinfection), ethylene glycol (preservation of tissue plasticity), hot water (softening of the tissues), as well as formalin (disinfection, tissue fixative, and embalming)16,17,18,19,20. - (Optional) Before injection of the latex milk, inject 20–30 mL of diluted ammonia to clear the vessels.
- Carefully mix a red color agent with 150 mL of latex milk (see Table of Materials).
- Inject the mixture using an air pressure pump system for about 10–15 min into the CCAs.
NOTE: Take care during the mixing and injection of the latex milk to keep it as bubble-free as possible. If a bubble is present, a gap develops inside the vessel, which might result in rupture of the artery during surgical intervention performed on the cadaver. - During the injection, use the same pressure or slightly higher to completely distribute the latex milk into the finer vessels in the orofacial region.
- After complete filling and staining of the CCAs and the subbranches, obstruct them with surgical vessel clamps to prevent the exiting of the injected latex milk.
- Embalm the specimens for approximately 6–8 months in Thiel's solution inside chrome vanadium corrosion resistant steel tanks.
- Keep the corpses in zipper polyethylene plastic bags for another 6 months with antifungal and preservative agents such as chlorocresol. During this period, the excess amount of extracellular liquid is pushed out of the body, and the specimens become ready for dissection.
NOTE: Following the completion of the fixation period, the tissues and the blood vessels of the maxillofacial region are ready and capable of withstanding the rigidity of dissection. The stained arteries can be analyzed by CT scans. Some of the subbranches in the palate, vestibule, tongue, and maxillofacial region are observable without removal of the mucosa. Finally, specimens are dissected by using 2.5x magnification loupes and Nr. 15C surgical blades. Mucosal elevation can be performed by dissecting the stained arteries layer by layer, and the course of the vessels and their variations can be macroscopically assessed.
Results
Using Thiel’s solution as a fixative for human corpses, training in surgical techniques and invasive clinical procedures becomes possible. One of the critical advantages of Thiel's solution is the maintenance of the natural color of soft and hard tissues. Thiel’s solution is less irritating compared to many other fixatives, such as formaldehyde. Its use results in flexibility of the temporomandibular joints and the masticatory and suprahyoid muscles, which facilitates dentoalveolar cadaver surgeries. Also, the texture of the neurovascular bundles is kept unspoiled and helps make practicing surgical techniques more convenient for the surgeon. Combining Thiel’s embalming method and subsequent latex milk injection is a clear, straightforward approach to prepare odor-free, fresh cadavers with natural consistency, in which the arteries of the maxillofacial region, especially the oral vestibule and the palate, are injected with a red color latex (Figure 3, Figure 4). The presence of radiopaque material in the latex milk allows the clinician to perform radiographical (CT) analysis (Figure 5) of the head specimen. Subsequently, the pathway of the vessels can be observed macroscopically to understand where and how to create an incision or a flap during implant placement, sinus floor elevation, or periodontal and maxillofacial surgeries. As a result of understanding the basic solid anatomy, acceptable postoperative angiogenesis and primary wound healing might be achieved when performing surgery.
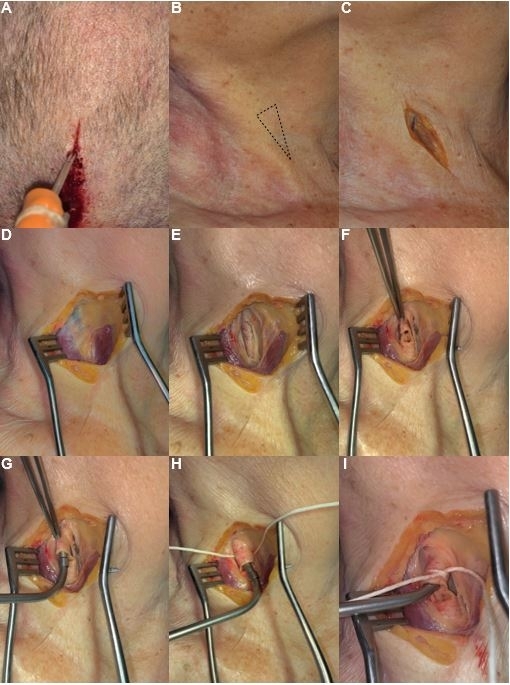
Figure 1: Step by step protocol for Thiel's solution and latex milk injection. (A) Catheter placement in the superior sagittal sinus for injection of Thiel's solution. (B) Localization of the carotid trigone. (C) Designing an incision parallel to the sternocleidomastoid muscle. (D) Separation of skin contacts and removal of cervical adipose tissue. (E) Dissection of the carotid sheath and cervical fascia between the sternocleidomastoid muscle (laterally) and inferior belly of the omohyoid muscle (medially). (F) Preparation of a dissected common carotid artery. (G) Adjustment of the catheter in a common carotid artery. (H) Fixation and isolation of the catheter. (I) Isolated catheter ready for injection of the Thiel's solution and red color latex milk injection. Please click here to view a larger version of this figure.
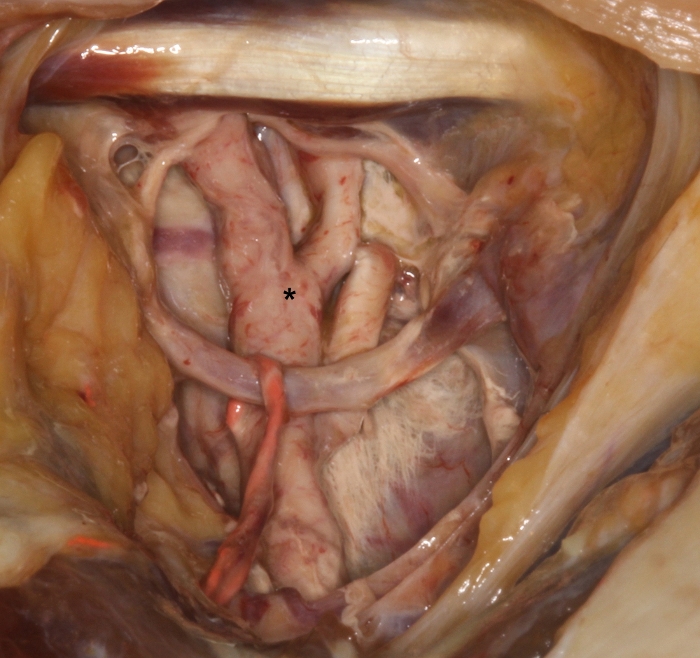
Figure 2: Demonstration of hardened red colored latex milk in the external carotid artery (*) with its subbranches at the upper part of the carotid trigone. Please click here to view a larger version of this figure.
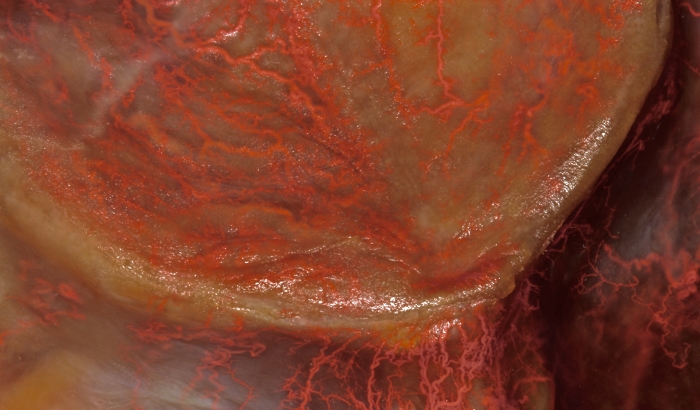
Figure 3: Vascular survey of the palate. Palatal mucosal distribution of the greater palatine and nasopalatine branches are visible macroscopically. Please click here to view a larger version of this figure.
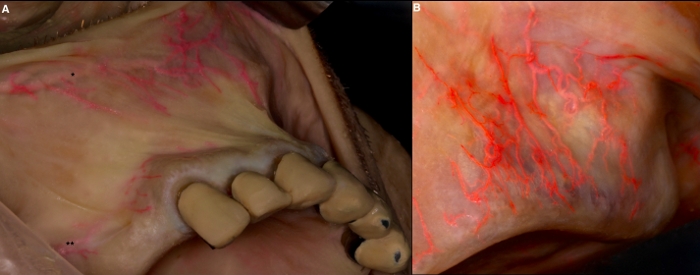
Figure 4: Vascular survey of the oral vestibule. (A) Horizontal path of superior labial (*) and posterior superior alveolar (**) arteries with their subbranches supplying the upper vestibular mucosa. (B) Vertical orientation of the arteries in the maxillary vestibule. Please click here to view a larger version of this figure.
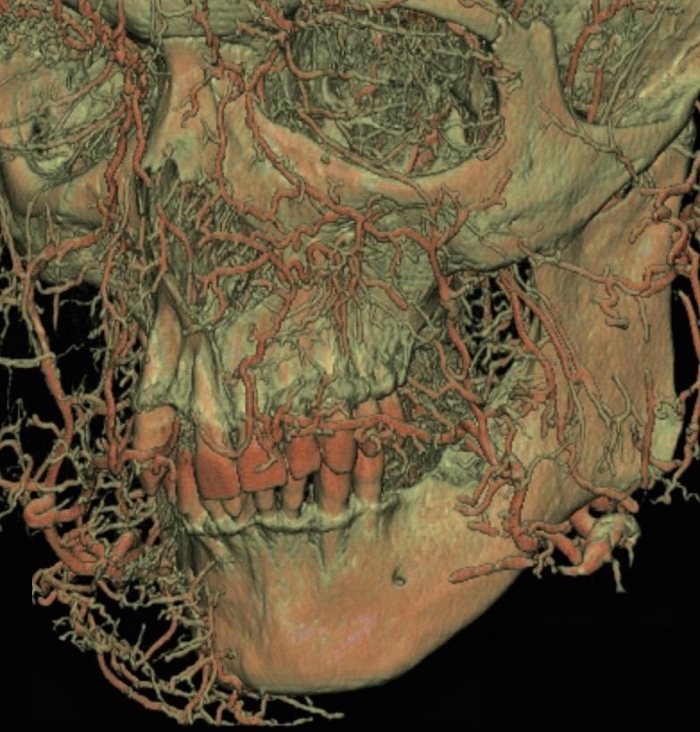
Figure 5: CT images of the various arterial pathways injected with the red colored latex milk in the maxillofacial region. Please click here to view a larger version of this figure.
Discussion
The unique combination of Thiel's embalming and latex milk injection methods enable the oral-maxillo-facial surgeons and periodontists to practice on fresh specimens while observing the directions of different vessels4,19,20. However, there might be some limitations with the staining of small-diameter vessels due to blockage by clots or not using enough pressure to push the material into the small arteries during the procedure.
The Thiel embalming method contains crucial vital advantages. For example, the cadavers are preserved in their natural color16,17,20. Due to the maintenance of resilience and elasticity of tissues20,21, this is an excellent method to demonstrate surgery and oral surgical interventions19,22,23. Because the Thiel method is odorless and less toxic compared to other fixation methods such as formaldehyde, it makes specimen preparation more convenient16,17,18,20. In 2011, Benkhedra investigated the histological features of unfixed and fixed skeletal muscle fibers comparing Thiel's solution and formalin24. With the Thiel method, the arrangement of the collagen fibers and the integrity of the muscles remained aligned compared to the other method24. Furthermore, ultrasound-guided research has reported that the Thiel embalming method gives more realistic results for radiologists and anesthesiologists25.
There is insufficient information regarding the precise route and anastomosis of the vessels in the oral mucosa, which may produce an improper flap design in surgery. The latex milk injection stains the blood vessels, and as a result, they become visible4,11. This facilitates the dissection of soft and hard tissues by discovering their anatomical and physiological relation to the vessels. Another essential characteristic of latex milk is that it is radiopaque due to the presence of lead(II,IV) oxide. Therefore, the pathway of the vessels can be observed three-dimensionally by CT analysis, which allows the operator to plan surgery according to an anatomical and vascular survey. As a consequence, an appropriate incision can be designed that prevents nerve damage, excessive bleeding26, and postoperative complications such as tissue necrosis and delayed primary wound healing27 in different oral surgical interventions.
Disclosures
The authors have no conflict of interest.
Acknowledgements
The authors would like to specially thank to all co-workers of the Department of Macroscopical and Clinical Anatomy of Graz University; the co-workers of the Department of Anatomy, Histology and Embryology, Semmelweis University; as well as Prof. Dr. Péter Windisch at the Department of Periodontology, Semmelweis University for their work and support.
Materials
| Name | Company | Catalog Number | Comments |
| Nr. 20 surgical blade | Braun, Tuttlingen, Germany | ||
| 2.5x magnification loupes | Harmonycom | ||
| 4-chloro-3-methylphenol | Jäckle Chemie Austria GmbH, Vienna Austria Lactan Chemicals, Graz Austria Brenntag Austria GmbH, Vienna, Austria | ||
| Air pressure pump system | Heiz-Hofstätter, Graz, Austria | ||
| Ammonium nitrate | Jäckle Chemie Austria GmbH, Vienna Austria Lactan Chemicals, Graz Austria Brenntag Austria GmbH, Vienna, Austria | ||
| Block End Serrated Dressing Forceps | VETisco, Chesterfield, England | ||
| Boric acid | Jäckle Chemie Austria GmbH, Vienna Austria Lactan Chemicals, Graz Austria Brenntag Austria GmbH, Vienna, Austria | ||
| Chlorocresol | Jäckle Chemie Austria GmbH, Vienna Austria Lactan Chemicals, Graz Austria Brenntag Austria GmbH, Vienna, Austria | ||
| Chrome Vanadium Corrosion Resistant Steel tanks | |||
| Computed tomography (CT) | Siemens, Emotion, Munich, Germany | ||
| Diluted ammonia | Jäckle Chemie Austria GmbH, Vienna Austria Lactan Chemicals, Graz Austria Brenntag Austria GmbH, Vienna, Austria | ||
| Drill (PSR, 10.8,LI-2) | Bosch AG, Gerlingen, Germany | ||
| Ethylene glycol | Jäckle Chemie Austria GmbH, Vienna Austria Lactan Chemicals, Graz Austria Brenntag Austria GmbH, Vienna, Austria | ||
| Formalin | Jäckle Chemie Austria GmbH, Vienna Austria Lactan Chemicals, Graz Austria Brenntag Austria GmbH, Vienna, Austria | ||
| Hot water | ——————— | ||
| Latexmilch (latex milk) | Creato, Zitzmann Zentrale, Baden, Germany | ||
| Metal catheter | Grall Enterprise, Graz, Austria | ||
| Nr. 15 surgical blade | Braun, Tuttlingen, Germany | ||
| Nr. 15C surgical blade | Braun, Tuttlingen, Germany | ||
| Pintasol red E-L3 mix paste | Mixol-products Diebold GmbH, Kirchheim, Germany | Color agent | |
| Potassium nitrate | Jäckle Chemie Austria GmbH, Vienna Austria Lactan Chemicals, Graz Austria Brenntag Austria GmbH, Vienna, Austria | ||
| Sodium sulfite | Jäckle Chemie Austria GmbH, Vienna Austria Lactan Chemicals, Graz Austria Brenntag Austria GmbH, Vienna, Austria | ||
| Surgical vessel clamp | Grall, Graz Austria | ||
| Weitlaner Retractor (Self-Retaining) 140mm 3/4 Teeth | VETisco, Chesterfield, England | ||
| Zipper polyethylene bags (plastics) | Wettlinger, Kunststoffe, Vienna, Austria |
References
- Kleinheinz, J., Büchter, A., Kruse-Lösler, B., Weingart, D., Joos, U. Incision design in implant dentistry based on vascularization of the mucosa. Clinical Oral Implants Research. 16 (5), 518-523 (2005).
- Koymen, R., et al. Flap and incision design in implant surgery: clinical and anatomical study. Surgical and Radiologic Anatomy. 31 (4), 301-306 (2009).
- Choi, J., Park, H. S. The clinical anatomy of the maxillary artery in the pterygopalatine fossa. Journal of Oral and Maxillofacial Surgery. 61 (1), 72-78 (2003).
- Shahbazi, A., et al. Analysis of blood supply in the hard palate and maxillary tuberosity-clinical implications for flap design and soft tissue graft harvesting (a human cadaver study). Clinical Oral Investigations. 23 (3), 1153-1160 (2019).
- Pilsl, U., Anderhuber, F., Neugebauer, S. The Facial Artery-The Main Blood Vessel for the Anterior Face. Dermatologic Surgery. 42 (2), 203-208 (2016).
- Akolkar, A. R., et al. Bleeding control measures during oral and maxillofacial surgical procedures: A systematic review. Journal of Dental Research and Review. 4 (4), 79-89 (2017).
- Arnold, F., West, D. C. Angiogenesis in wound healing. Pharmacology & Therapeutics. 52 (3), 407-422 (1991).
- Yu, Q. X., et al. The microvasculature of human oral mucosa using vascular corrosion casts and India ink injection. I. Tongue papillae. Scanning Microscopy. 6 (1), 255-262 (1992).
- Yu, Q. X., Pang, K. M., Ran, W., Philipsen, H. P., Chen, X. H. The microvasculature of human infant oral mucosa using vascular corrosion casts and india ink injection. II. Palate and lip. Scanning Microscopy. 8 (1), 133-139 (1994).
- Bergeron, L., Tang, M., Morris, S. F. A review of vascular injection techniques for the study of perforator flaps. Plastic and Reconstructive Surgery. 117 (6), 2050-2057 (2006).
- Alvernia, J. E., Pradilla, G., Mertens, P., Lanzino, G., Tamargo, R. J. Latex injection of cadaver heads: technical note. Neurosurgery. 67, 362-367 (2010).
- Haenssgen, K., Makanya, A. N., Djonov, V. Casting Materials and their Application in Research and Teaching. Microscopy and Microanalysis. 20 (2), 493-513 (2014).
- Jaung, R., Cook, P., Blyth, P. A comparison of embalming fluids for use in surgical workshops. Clinical Anatomy. 24 (2), 155-161 (2011).
- Balta, J. Y., Lamb, C., Soames, R. W. A pilot study comparing the use of Thiel- and formalin-embalmed cadavers in the teaching of human anatomy. Anatomical Sciences Education. 8 (1), 86-91 (2015).
- Hayashi, S., et al. Saturated salt solution method: a useful cadaver embalming for surgical skills training. Medicine. 93 (27), 196 (2014).
- Thiel, W. The preservation of the whole corpse with natural color. Annals of Anatomy. 174 (3), 185-195 (1992).
- Thiel, W. An arterial substance for subsequent injection during the preservation of the whole corpse. Annals of Anatomy. 174 (3), 197-200 (1992).
- Thiel, W. Ergänzung für die Konservierung ganzer Leichen nach W. Thiel. Annals of Anatomy. 184 (3), 267-269 (2002).
- Okada, R., et al. Thiel's method of embalming and its usefulness in surgical assessments. Nihon Jibiinkoka Gakkai Kaiho. 115 (8), 791-794 (2012).
- Ottone, N. E., Vargas, C. A., Fuentes, R., Del Sol, M. Walter Thiel's Embalming Method. Review of Solutions and Applications in Different Fields of Biomedical Research. International Journal of Morphology. 34 (4), 1442-1454 (2016).
- Peuker, E. T., Werkmeister, R., Pera, F., Joos, U., Filler, T. J. Surgical procedures in mouth, jaw and facial surgery in Thiel embalmed body donors. Mund Kiefer Gesichtschir. 5 (2), 141-143 (2001).
- Wolff, K. D., Kesting, M., Mücke, T., Rau, A., Hölzle, F. Thiel embalming technique: a valuable method for microvascular exercise and teaching of flap raising. Microsurgery. 28 (4), 273-278 (2008).
- Hölzle, F., et al. Thiel embalming technique: a valuable method for teaching oral surgery and implantology. Clinical Implant Dentistry and Related Research. 14 (1), 121-126 (2012).
- Benkhadra, M., et al. Flexibility of Thiel's embalmed cadavers: the explanation is probably in the muscles. Surgical and Radiologic Anatomy. 33 (4), 365-368 (2011).
- Benkhadra, M., et al. Comparison of fresh and Thiel's embalmed cadavers according to the suitability for ultrasound-guided regional anesthesia of the cervical region. Surgical and Radiologic Anatomy. 31 (7), 531-535 (2009).
- Lamas Pelayo, J. Intraoperative complications during oral implantology. Medicina Oral, Patología Oral y Cirugía Bucal. 13 (4), 239-243 (2008).
- Lim, G., Lin, G. H., Monje, A., Chan, H. L., Wang, H. L. Wound Healing Complications Following Guided Bone Regeneration for Ridge Augmentation: A Systematic Review and Meta-Analysis. The International Journal of Oral & Maxillofacial Implants. 33 (1), 41-50 (2018).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved