A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Multipronged Phenotyping Approaches to Characterize Sugarcane Root Systems
In This Article
Summary
Characterizing root system traits is one of the areas of research that is still in its infancy, particularly in sugarcane. Integrating multiple approaches to precisely phenotype sugarcane roots leads to comprehensive and holistic results, enabling the utilization of identified traits and mechanisms for conventional and molecular breeding.
Abstract
Roots are the primary conductors of water and nutrients and play a vital role in sustaining growth and yield under stressful environments. The study of plant roots poses methodological difficulties in in situ assessment and sampling, which is especially true for sugarcane (Saccharum spp.). Traditional methods during the 1920s documented the genotypic variation in sugarcane root systems, after which few studies were reported on sugarcane root traits per se until recently. In addition to morphology, rhizosphere characteristics, including allelopathic effects and/or affinity for microbial symbiosis, determine plant establishment and survival.
Ultimately, root systems define the above-ground productivity of sugarcane. With the impetus for climate-resilient varieties, it is becoming more relevant to explore and utilize the variability in root system traits of sugarcane. This paper describes multipronged approaches for sugarcane root phenotyping, including field excavation by trench sampling, the use of a root core sampler, raised platforms for root sampling, and raising plants under hydroponic culture, employed by a team of scientists at the Indian Council of Agricultural Research-Sugarcane Breeding Institute (ICAR-SBI).
Field excavation by trench sampling is imperative to assess the plant roots in their natural growing environment. The use of raised platforms simulating field conditions and a root core sampler are alternative approaches, with a considerable reduction in time, uniform sample size, and less loss of root material. Hydroponic plant culture allows the study of morphology, anatomical features, and rhizosphere biology, including the exudation of organic compounds and microbial interactions. Data generated from different experiments using diverse sampling methods add to the wealth of information on the root system traits of sugarcane.
Introduction
Sugarcane (Saccharum spp.), an important food and bioenergy source, is a significant industrial crop suited for cultivation in many countries with tropical and subtropical climatic conditions. Owing to the C4 photosynthetic pathway for carbon assimilation, sugarcane is highly productive, efficiently using farm inputs such as water and fertilizers. Post-harvest processing of sugarcane yields economically valuable products such as sugar and jaggery, alongside its byproducts-molasses, ethanol, and energy. Sugarcane is produced by nearly 100 countries over an area of 25.97 Mha, which is approximately 1.5% of total arable land. India alone contributes to 16% of world sugarcane production (approximately 306 Mt), with an average productivity of 70 t·ha−1 1. The major abiotic stresses drastically affecting sugarcane production include water deficits, water logging, temperature extremes, and soil properties such as nutrient deficiency, salinity, sodicity, and alkalinity. The biggest challenge for developing abiotic stress-tolerant crops is to pinpoint specific traits that confer a substantial yield advantage under stress conditions.
Several aspects of sugarcane physiology are poorly understood, including the root-shoot relationship, which drastically affects cane productivity. Sugarcane root is not as well studied as shoots, although the different types, such as sett roots, shoot roots, and adult roots, may be developmentally distinct with varying functions. Genotypic differences have been observed with regard to the number and length of sett roots emerging during germination2. Sett roots are implicated in the germination of sugarcane buds, ensuring early crop establishment, and are later replaced by shoot roots, which are robust, emerging from the base of the developing shoot3. Fine branches observed in the sett roots help in anchoring the young plants and aid in the absorption of water and nutrients until they are replaced by shoot roots. Similar to sett roots, shoot roots also arise from the root primordia present in the lower, unexpanded internodes of the cane.
As shoot roots persist in the plant for a longer duration, they are 4x-10x thicker than sett roots. Shoot roots constitute the sole root system of sugarcane, with an important role in further growth and development. The vigor of the shoot roots is positively associated with the overall vegetative vigor of the plant. The continuous development of roots resulting from the turnover of sett roots and shoot roots gives rise to the "adult root system" of sugarcane, which is ever-adapting to the prevailing environmental conditions. In general, a deeper, more prolific root system makes more water and nutrients available for the crop than does a shallower distribution of roots. Periodic dissections revealed that, when the soil moisture content was high, shallow root systems were observed, whereas a much deeper root system developed as the water table dropped2. The root system in sugarcane remains active even after harvesting of the crop, contributing to the growth of the ratoon crop until new shoot roots emerge from underground buds4. Root angle and the level of root branching are two important factors determining the volume of soil explored by plant roots. Root angle, a genotypic trait, may be altered through conventional breeding or molecular approaches to improve tolerance to biotic and abiotic stresses. On the contrary, the level of root branching is mostly influenced by the environment, necessitating periodic monitoring of root development and its response to localized soil conditions.
Anatomical features of sugarcane roots have been examined to ascertain differences with regard to genotype and environment. The anatomy of sett roots in sugarcane resembles that of mature roots in other grasses such as maize, wherein the cortex comprises well-differentiated cell layers in a regular pattern. The endodermis is suberized, followed by a single-layered pericycle. Metaxylem elements are the main conductors or water and nutrient ions, radially arranged and interspersed with groups of phloem, the latter comprising a sieve element with two companion cells. The large central mass of undifferentiated cells forms the root pith. Distinct anatomical features of sugarcane cultivars correspond to root hydraulic properties, thereby influencing water movement. Early studies on the differences in the root anatomical traits of sugarcane revealed that, under low moisture stress conditions, pronounced thickening of the cell wall was observed in the layers immediately inside of the endodermis, between the pith and the vascular region, and around the vessels5. Such thickened cells may be an adaptation to prevent the backward flow of sap and for mechanical strength during stress.
Some important traits implicated in the drought resistance of sugarcane include the relative thickness and number of exodermal layers, the ratio of cortex to stele, intercellular spaces in the cortex, and thickened root hair tips. The ratios of the area occupied by cortical cells to the area occupied by the stellar tissues of shoot roots are significantly different among sugarcane cultivars, with wide variability with respect to the area of the stele6. The hydraulic conductivity of sugarcane roots is related to the size and number of metaxylem elements in the shoot roots. Hydrophobic cell layers within the root are likely to define zones of apoplastic water movement. Suberized Casparian bands are found in the endodermis and in the hypodermis (termed as exodermis), which serve as hydrophobic barriers. The disintegration of cortical cells leads to the formation of lysigenous aerenchyma in older roots and in plants subjected to hypoxic conditions, irrespective of developmental age. The formation of aerenchyma during waterlogging stress is correlated with the maintenance of growth in resistant varieties7.
The morphology and anatomy of Erianthus arundinaceus [Retzius] Jeswiet (genera related to Saccharum spp.) roots are implicated in its strong tolerance to environmental stresses8. Erianthus arundinaceus roots exhibit nodal roots distributed at steep angles, with dense roots hairs to facilitate the uptake of water and nutrient ions from deeper soil zones. The deep-root system consists of many nodal roots growing with steep growth angles. The diameter of the nodal roots correlates with the size and number of large xylem vessels, the former varying widely from 0.5 mm to 5.0 mm. These nodal roots also form a rhizomatous sheath, with a hypodermis showing lignified sclerenchyma in the outer cortex (exodermis), lysigenous aerenchyma in the mid-portion of the cortex, and starch granules in the stele. In addition to architecture and morphological traits, root-exuded organic compounds play an important role in determining plant germination, establishment, and survival, with plausible allelopathic effects and/or affinity for microbial symbiosis.
Root enzymatic activity and the finer details of the morphology, including root cap pigmentation and rejuvenation potential upon injury, were documented in sugarcane varieties grown under hydroponic culture9. Root growth shows a highly plastic response to changes in the soil environment, both in terms of the form and size of the root system. The most efficient sugarcane variety would be one that has few or an optimal number of shoots, with a correspondingly lower number of roots, aiding better survival during stressful conditions. The systematic study of the root system should, thus, form an important component of any crop improvement program10. The majority of the experiments focusing on roots rely mostly on developmental aspects, while a focus on functional plasticity is often lacking11. Apart from the structural distribution, functional root plasticity plays a crucial role in survival under stress and would, therefore, support breeders in their efforts to include root system traits in the selection pipeline for abiotic stress tolerance and improve the robustness of sugarcane.
Considering its importance in sustaining growth and yield under stressful environments, it is essential to explore and utilize the inherent variability in root system traits of sugarcane. An emphasis on the selection of component traits and mechanisms imparting superior root systems is the way forward for better crop performance under changing climatic conditions. Phenotypic evaluation is a long and costly process; however, the integration of multipronged approaches would add tremendous value to its utility in crop improvement. In this manuscript, four different approaches for root phenotyping in sugarcane are described, each with its own set of merits and demerits, implying that a concerted effort is required to arrive at comprehensive and holistic results.
Protocol
1. Field excavation by trench sampling
- Raise commercial sugarcane hybrids (hereafter called "varieties") in the field from two-budded setts planted at a row spacing of 120 cm, with 90 cm within rows, following the recommended package of practices (POP) to ensure good crop establishment and growth.
- At the end of the maturity phase of the crop, employ an excavator to dig a trench (1.5 m deep and 1.0 m wide) in the field. Through continuous water jetting, ensure that the soils from root zones are cleared without damaging the roots (Figure 1).
- When the adhering soil loosens, uproot the cane along with the root system and take it to the laboratory for manual measurement of the number of roots, root length, volume, and weight.

Figure 1: Trench sampling method for the field excavation of roots. (A) Sideways trench dug along the field, (B) water jetting, and (C) inner view showing the depth of the trench. Please click here to view a larger version of this figure.
2. Root core sampler to reduce sampling errors
- Use a cylindrical root core sampler of 61 cm height and 16 cm diameter weighing 8 kg, fabricated using mild steel (MS) material for sugarcane root sampling in the field. Provide a sharp edge at the bottom edge of the sampler to facilitate easy penetration while inserting it into the soil. Provide the top with collars of 3 cm diameter to lift the sampler (Figure 2).
- Raise sugarcane varieties in the field from two-budded setts planted at a row spacing of 120 cm, with 90 cm within rows, following the recommended POP to ensure good crop establishment and growth.
- At the maturity phase of the crop, fasten the top edge of the sampler to the primary shoot/cane and hammer continuously to reach the desired soil depth (45 cm). Lift the entire soil mass into the sampler and wash carefully under running water to separate the adhering roots.
- After thorough washing of the roots, record the volume, surface area, length, and weight by manual measurement, as well as by spreading the roots on transparent trays to scan and analyze the corresponding digitized images using the referenced software (see the Table of Materials).
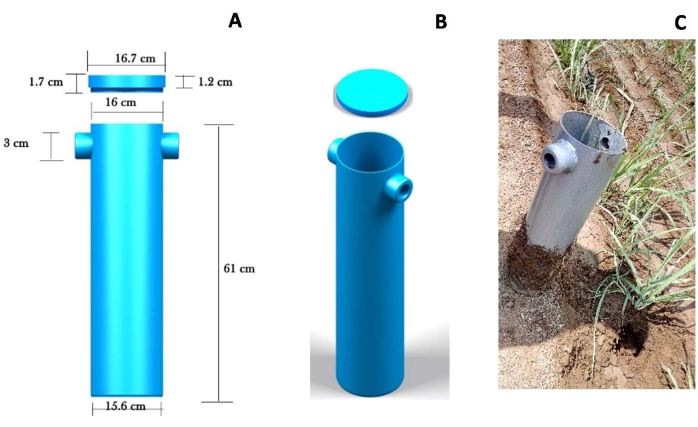
Figure 2: Root core sampler. (A) Dimensions, (B) top view, and (C) site of sampling. Please click here to view a larger version of this figure.
3. Root phenotyping structure to facilitate sampling at different phenophases
- Construct a root phenotyping structure comprising three adjacent compartments of dimensions 4.5 m x 10.1 m for sampling of the sugarcane roots, with provisions for manually dismantling the side walls to reveal the underground root system (up to a depth of 80-100 cm) (Figure 3). Construct the side walls with precast slabs of dimensions 1.8 m in length, 30 cm in width, and 4 cm in thickness.
- Fill and compact the structure with field soil, leaving a headspace of ~20 cm, with adequate drainage holes to facilitate soil aeration.
- Sow the bud chips of germplasm clones comprising Saccharum officinarum L., Saccharum spontaneum L., Saccharum barberi Jesw., Saccharum sinense Roxb., and Saccharum robustum Brandes and Jeswiet ex Grassl. (hereafter called "Saccharum spp. clones") and allow them to germinate for 30 days in protrays comprising rooting media (red soil: farm yard manure: sand = 2:2:1). Transplant uniform and healthy settlings to the structure at a row spacing of 90 cm, with 60 cm within rows, following the recommended POP to ensure good crop establishment and growth.
- During the formative (60-120 days after planting [DAP]) and grand growth (120-150 DAP) phases, manually remove the side walls made of the precast slabs, followed by continuous spraying of the water jet to expose the roots.
- Uproot the entire root system and take it to the laboratory for manual measurement of the length, volume, and weight, and spread the roots on transparent trays to scan and analyze the corresponding digitized images in the referenced software (see the Table of Materials).
- Impose drought stress by withholding irrigation in one of the compartments, and plug the drainage holes in the second compartment to maintain soil saturation to simulate waterlogging stress. Irrigate the third compartment according to the recommended POP to maintain the field capacity and to serve as a control.
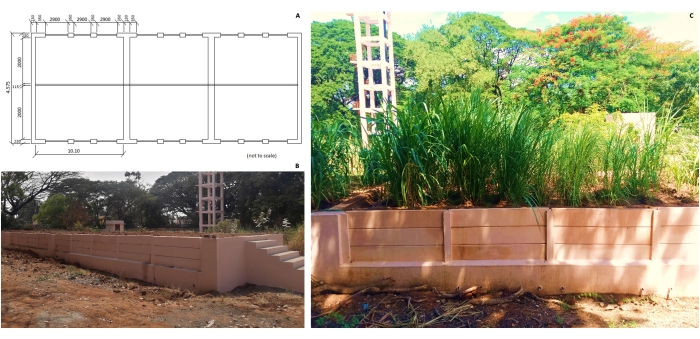
Figure 3: Root phenotyping structure. (A) Dimensions, (B) overview of the three compartments, and (C) view of one compartment. Please click here to view a larger version of this figure.
4. Hydroponic culture of plants to study rhizosphere biology
- Fabricate an in-house hydroponic system in an environment-controlled glasshouse conducive for growing sugarcane to study the finer details of root biology. Add ~15 L of modified Hoagland's nutrient solution (Table 1) to glass tanks of dimensions 20 cm x 20 cm x 50 cm, with aeration provided by aquarium pumps (Figure 4).
- Sow the bud chips of sugarcane varieties and Saccharum spp. clones and allow them to germinate for 30 days in protrays comprising composted coir pith. Transplant uniform and healthy settlings to hydroponic tanks at the frequency of three settlings per tank, taking care to place the entire root in the nutrient solution. Cover the tanks with black cloth to ensure that roots are not exposed to light. Use a plastic mesh (20 cm x 20 cm) at the brim of the glass tanks to support the plants upright.
- At the end of the germination phase (60 days), collect the root exudates by immersing the roots of intact plants in 50 mL of trap solution (sterile double-distilled water) for 4 h (0800 h to 1200 h) during the time of peak photosynthetic activity. Filter the collected solution through Whatman filter paper, then pass it through glass columns filled with anion-exchange followed by cation-exchange resins12. Evaporate the eluted fractions to dryness and store at -20 °C until further processing.
- Analyze the processed root exudate samples by HPLC for the determination of organic acids12 and by spectrophotometry for the estimation of total phenolics13, proteins14, sugars15, and amino acids16 according to standard protocol.
- Monitor the root growth at weekly intervals to record the root tip pigmentation and root hair density. Assess the activity of the enzymes, peroxidase17 and superoxide dismutase18, and total phenolic content19 at the 3rd month according to standard protocol.
- Assess the response to root injury by inflicting a longitudinal slice in the primary root up to the root tip using a sterile surgical blade, and monitor the changes periodically.
| Chemical | Final concentration |
| Potassium nitrate | 0.608 g·L-1 |
| Calcium nitrate | 1.415 g·L-1 |
| Potassium dihydrogen phosphate | 0.164 g·L-1 |
| Magnesium sulphate | 0.560 g·L-1 |
| EDTA-ferric monosodium salt | 6.00 g·250L-1 |
| Boric acid | 1.43 g·250L-1 |
| Manganese chloride tetrahydrate | 0.91 g·250L-1 |
| Zinc sulphate | 0.11 g·250L-1 |
| Cupric suphate | 0.04 g·250L-1 |
| Molybdic acid | 0.01 g250L-1 |
Table 1: Composition of the modified nutrient solution for the hydroponic culture of sugarcane.
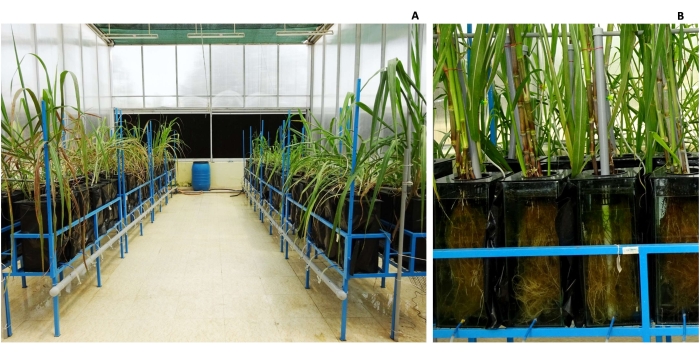
Figure 4: Hydroponics setup. The setup (A) customized for growing sugarcane and (B) 5-month-old crop (black cloth removed for photography purpose only). This figure has been modified from Hari et al.9. Please click here to view a larger version of this figure.
Results
Variation in the root morphological traits of sugarcane varieties
Representative images of the root system in Co 62175, excavated from the field by trench sampling and grown in a hydroponic setup, are presented in Figure 5A,B. Long roots (~100 cm) were observed in the varieties Co 62175 and Co 99006, while Co 99006 recorded the highest root weight (127 g·clump−1). Root traits were recorded using a root core sampler, which revealed...
Discussion
Root systems define the above-ground productivity of sugarcane, necessitating that all its facets be explored and understood thoroughly for the development of climate-resilient varieties. A team of scientists at ICAR-SBI comprising plant physiologists, a microbiologist, an agricultural engineer, a biochemist, and plant breeders employed multipronged approaches for sugarcane root phenotyping, including field excavation by trench sampling, the use of a root core sampler, raised platforms for root sampling, and raising plan...
Disclosures
All authors declare that there are no conflicts of interest.
Acknowledgements
The authors acknowledge the infrastructure and support extended by the Director, ICAR-Sugarcane Breeding Institute, Coimbatore, for establishing root phenotyping facilities for sugarcane. Funding provided by the Science and Engineering Research Board, Department of Science and Technology, Government of India, in the form of Early Career Research Award to KV (ECR/2017/000738), is duly acknowledged. The authors acknowledge Brindha, Karpagam, Rajesh, Sivaraj, and Amburose for their assistance in generating data in a meticulous manner.
Materials
| Name | Company | Catalog Number | Comments |
| Aeration pump with pipeline accessories | Purchased from local sources | NA | Used for hydroponic culture of sugarcane |
| Boric acid | Sisco Research Laboratories, India | 80266 | Preparation of modified Hoagland's solution |
| Calcium nitrate | Central Drug House, India | 27606 | Preparation of modified Hoagland's solution |
| Composted coir pith | Purchased from local sources | NA | Used for germinating sugarcane setts |
| Cupric sulphate | Sisco Research Laboratories, India | 38869 | Preparation of modified Hoagland's solution |
| DEAE-cellulose | Sisco Research Laboratories, India | 10529 | anion exchange resin for processing of root exudates |
| EDTA-ferric monosodium salt | Sisco Research Laboratories, India | 59389 | Preparation of modified Hoagland's solution |
| Farm yard manure | Purchased from local sources | NA | Used for germinating sugarcane setts |
| Glass tanks | Fabricated in-house | NA | Used for hydroponic culture of sugarcane |
| HPLC | Agilent Technologies | 1200 Infinity | Quantification of organic acids in root exudates |
| Magnesium sulphate | Sisco Research Laboratories, India | 29117 | Preparation of modified Hoagland's solution |
| Manganese chloride | Sisco Research Laboratories, India | 75113 | Preparation of modified Hoagland's solution |
| Molybdic acid | Sisco Research Laboratories, India | 49664 | Preparation of modified Hoagland's solution |
| Potassium dihydrogen phosphate | Central Drug House, India | 29608 | Preparation of modified Hoagland's solution |
| Potassium nitrate | Central Drug House, India | 29638 | Preparation of modified Hoagland's solution |
| Protrays | Fabricated in-house | NA | Used for germinating sugarcane setts |
| Red soil | Purchased from local sources | NA | Used for germinating sugarcane setts |
| Root core sampler | Fabricated in-house | NA | Used for in situ root sampling |
| Sand | Purchased from local sources | NA | Used for germinating sugarcane setts |
| Seralite-120 | Sisco Research Laboratories, India | 14891 | cation exchange resin for processing of root exudates |
| Supporting frame | Purchased from local sources | NA | Used for hydroponic culture of sugarcane |
| Water motor pump | Purchased from local sources | NA | Used for hydroponic culture of sugarcane |
| Whatman filter paper grade 1 | Universal Scientific | 1001090 | Processing of root exudates |
| WinRhizo PRO (software) | Regent Instruments Inc., Canada | STD4800 | Two-dimensional root scanner with software for analysis of roots |
| Zinc sulphate | Sisco Research Laboratories, India | 76455 | Preparation of modified Hoagland's solution |
References
- Venkatraman, T. S., Thomas, R. Studies of sugarcane roots at different stages of growth. Agricultural Journal of India. 23, 166-176 (1928).
- Smith, D. M., Inman-Bamber, N. G., Thorburn, P. J. Growth and function of the sugarcane root system. Field Crops Research. 92 (2-3), 169-183 (2005).
- Glover, J. The behaviour of the root-system of sugarcane at and after harvest. Proceedings of the South African Sugar Technologists Association. 42, 133-135 (1968).
- Venkatraman, T. S., Thomas, R. Sugarcane root systems: Studies in development and anatomy. Agricultural Journal of India. 17 (4), 416-418 (1922).
- Saliendra, N. Z., Meinzer, F. C. Genotypic, developmental and drought-induced differences in root hydraulic conductance of contrasting sugarcane cultivars. Journal of Experimental Botany. 43 (9), 1209-1217 (1992).
- Gilbert, R. A., Rainbolt, C. R., Morris, D. R., Bennet, A. C. Morphological responses of sugarcane to long-term flooding. Agronomy Journal. 99 (6), 1622-1628 (2007).
- Shiotsu, F., Abe, J., Doi, T., Gau, M., Morita, S. Root morphology and anatomy of field-grown Erianthus arundinaceus. American Journal of Plant Sciences. 6 (1), 103-112 (2015).
- Hari, K., Vasantha, S., Anna Durai, A., Brindha, C., Shruthi, P. Sugarcane root growth and development in hydroponics system. Journal of Sugarcane Research. 7 (2), 71-82 (2017).
- Matsuoka, S., Garcia, A. A. F. Sugarcane underground organs: Going deep for sustainable production. Tropical Plant Biology. 4, 22-30 (2011).
- Koevoets, I. T., Venema, J. H., Elzenga, J. T. M., Testerink, C. Roots withstanding their environment: Exploiting root system architecture responses to abiotic stress to improve crop tolerance. Frontiers in Plant Science. 7, 1335 (2016).
- Vengavasi, K., Pandey, R. Root exudation index: Screening organic acid exudation and phosphorus acquisition efficiency in soybean genotypes. Crop and Pasture Science. 67 (10), 1096-1109 (2017).
- Bray, H. G., Thorpe, W. V. Analysis of phenolic compounds of interest in metabolism. Methods of Biochemical Analysis. 1, 27-52 (1954).
- Lowry, O. H., Rosebrough, N. J., Farr, A. L., Randall, R. J. Protein measurement with the folin phenol reagent. The Journal of Biological Chemistry. 193 (1), 265-275 (1951).
- Hedge, J. E., Hofreiter, B. T. Determination of total carbohydrate by anthrone method. Carbohydrate Chemistry: Volume 17. , (1962).
- Moore, S., Stein, W. H. Polyphenol oxidase. Methods in Enzymology. Vol 468. , (1948).
- Malik, C. P., Singh, M. B. . Plant Enzymology and Histo-enzymology: A text manual. , (1980).
- Beauchamp, C., Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Analytical Biochemistry. 44, 276-287 (1971).
- Singleton, V. L., Rossi, J. A. Colorimetry of total phenolics with phosphomolybdic- phosphotungstic acid reagents. American Journal of Enology and Viticulture. 16, 144-158 (1965).
- Rege, R. D., Wagle, P. V. Problems of sugarcane physiology in the Deccan canal tract. III. The root-system. The Indian Journal of Agricultural Science. 2 (3), 356-373 (1940).
- Ryker, T. C., Edgerton, C. W. Studies on sugar cane roots. LSU Agricultural Experiment Station Reports. 223, (1931).
- Jensen, J. H. Some studies of root habits of sugar cane in Cuba. The Tropical Plant Research Foundation. , (1931).
- Kücke, M., Schmid, H., Spiess, A. A comparison of four methods for measuring roots of field crops in three contrasting soils. Plant and Soil. 172 (1), 63-71 (1995).
- Noordwijk, M., et al. Trench Profile Techniques and Core Break Methods. Root Methods: A handbook. , (2001).
- De Azevedo, M. C. B., Chopart, J. L., Medina, C. C. Sugarcane root length density and distribution from root intersection counting on a trench-profile. Scientia Agricola. 68 (1), 94-101 (2011).
- Nissen, T., Rodriguez, V., Wander, M. Sampling soybean roots: A comparison of excavation and coring methods. Communications in Soil Science and Plant Analysis. 39 (11-12), 1875-1883 (2008).
- Burridge, J. D., et al. An analysis of soil coring strategies to estimate root depth in maize (Zea mays) and common bean (Phaseolus vulgaris). Plant Phenomics. 2020, 3252703 (2020).
- Schroth, G., Kolbe, D. A method of processing soil core samples for root studies by subsampling. Biology and Fertility of Soils. 18, 60-62 (1994).
- Chandran, K., Nisha, M., Arun Kumar, R., Krishnapriya, V. Breeding varieties resistant to waterlogging. ICAR-SBI Annual Report 2016-17. , 128 (2016).
- Wu, Q., Wu, J., Zheng, B., Guo, Y. Optimizing soil-coring strategies to quantify root-length-density distribution in field-grown maize: Virtual coring trials using 3-D root architecture models. Annals of Botany. 121 (5), 809-819 (2018).
- Joshi, D. C., et al. Development of a phenotyping platform for high throughput screening of nodal root angle in sorghum. Plant Methods. 13, 56 (2017).
- Koyama, T., Murakami, S., Karasawa, T., Ejiri, M., Shiono, K. Complete root specimen of plants grown in soil-filled root box: Sampling, measuring, and staining method. Plant Methods. 17, 97 (2021).
- Gomathi, R., Gururaja Rao, P. N., Chandran, K., Selvi, A. Adaptive responses of sugarcane to waterlogging stress: An overview. Sugar Tech. 17, 325-338 (2014).
- Misra, V., et al. Morphological assessment of water stressed sugarcane: a comparison of waterlogged and drought affected crop. Saudi Journal of Biological Sciences. 27 (5), 1228-1236 (2020).
- Robinson, N., et al. Sugarcane genotypes differ in internal nitrogen use efficiency. Functional Plant Biology. 34 (12), 1122-1129 (2007).
- Arruda, B., et al. Biological and morphological traits of sugarcane roots in relation to phosphorus uptake. Journal of Soil Science and Plant Nutrition. 16 (4), 901-915 (2016).
- Sharma, S., Borah, P., Meena, M. K., Bindraban, P., Pandey, R. Evaluation of genotypic variation for growth of rice seedlings under optimized hydroponics medium. Indian Journal of Genetics and Plant Breeding. 78 (3), 292-301 (2018).
- Soumya, P. R., Singh, D., Sharma, S., Singh, A. M., Pandey, R. Evaluation of diverse wheat (Triticum aestivum) and triticale (× Triticosecale) genotypes for low phosphorus stress tolerance in soil and hydroponic conditions. Journal of Soil Science. 21, 1236-1251 (2021).
- Ganie, A. H., et al. Metabolite profiling and network analysis reveal coordinated changes in low-N tolerant and low-N sensitive maize genotypes under nitrogen deficiency and restoration conditions. Plants. 9 (11), 1459 (2020).
- Venkatraman, T. S., Thomas, R. Simple contrivances for studying root development in agricultural crops. Agricultural Journal of India. 18, 509-514 (1923).
- Clark, R. T., et al. High-throughput two-dimensional root system phenotyping platform facilitates genetic analysis of root growth and development. Plant, Cell and Environment. 36 (2), 454-466 (2013).
- Chopart, J. L., Rodrigues, S. R., Azevedo, M. C. B., Medina, C. C. Estimating sugarcane root length density through root mapping and orientation modelling. Plant and Soil. 313, 101-112 (2008).
- Chopart, J. L., Azevedo, M. C. B., Le Mezo, L., Marion, D. Functional relationship between sugarcane root biomass and length for cropping system applications. Sugar Tech. 12 (3-4), 317-321 (2010).
- De Silva, A. L. C., De Costa, W. A. J. M., Bandara, D. M. U. S. Growth of root system and the patterns of soil moisture utilization in sugarcane under rain-fed and irrigated conditions in Sri Lanka. Sugar Tech. 13 (3), 198-205 (2011).
- Otto, R., Silva, A. P., Franco, H. C. J., Oliveira, E. C. A., Trivelin, P. C. O. High soil penetration resistance reduces sugarcane root system development. Soil and Tillage Research. 117, 201-210 (2011).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved