16.2 : Buffers
A solution containing appreciable amounts of a weak conjugate acid-base pair is called a buffer solution, or a buffer. Buffer solutions resist a change in pH when small amounts of a strong acid or a strong base are added. A solution of acetic acid and sodium acetate is an example of a buffer that consists of a weak acid and its salt: CH3COOH (aq) + CH3COONa (aq). An example of a buffer that consists of a weak base and its salt is a solution of ammonia and ammonium chloride: NH3 (aq) + NH4Cl (aq).
How Buffers Work
To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium acetate. The presence of a weak conjugate acid-base pair in the solution imparts the ability to neutralize modest amounts of added strong acid or base. For example, adding a strong base to this solution will neutralize hydronium ion and shift the acetic acid ionization equilibrium to the right, partially restoring the decreased H3O+ concentration:
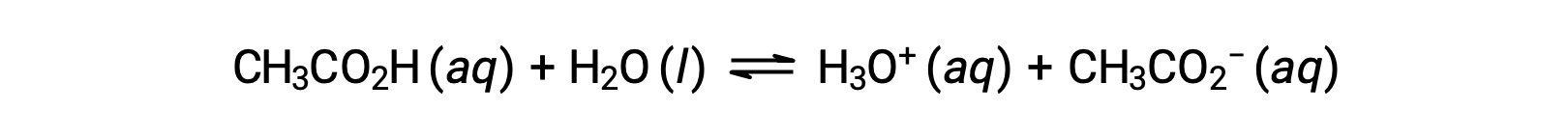
Likewise, adding a strong acid to this buffer solution will neutralize acetate ion, shifting the above ionization equilibrium right and returning [H3O+] to near its original value. Figure 1 provides a graphical illustration of the changes to the buffer solution when strong acid and base are added. The buffering action of the solution is essentially a result of the added strong acid and base being converted to the weak acid and base that make up the buffer's conjugate pair. The weaker acid and base undergo only slight ionization, as compared to the complete ionization of the strong acid and base. The solution pH, therefore, changes much less drastically than it would in an unbuffered solution.
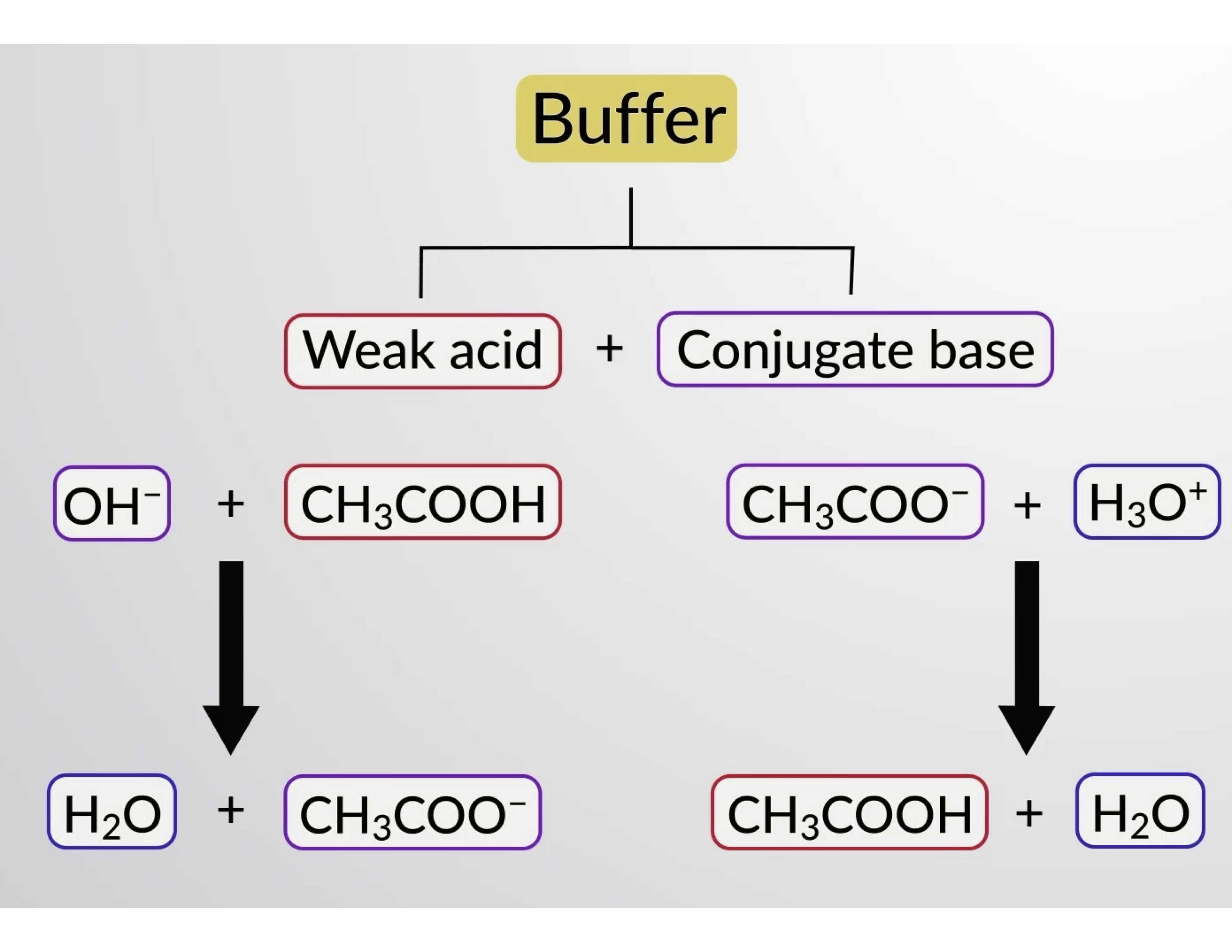
Figure 1. Buffering action in a mixture of acetic acid and acetate salt.
This text is adapted from Openstax, Chemistry 2e, Section 14.6: Buffers.
Z rozdziału 16:

Now Playing
16.2 : Buffers
Acid-base and Solubility Equilibria
163.2K Wyświetleń

16.1 : Wspólny efekt jonowy
Acid-base and Solubility Equilibria
40.8K Wyświetleń

16.3 : Równanie Hendersona-Hasselbalcha
Acid-base and Solubility Equilibria
67.9K Wyświetleń

16.4 : Obliczanie zmian pH w roztworze buforowym
Acid-base and Solubility Equilibria
52.5K Wyświetleń

16.5 : Skuteczność bufora
Acid-base and Solubility Equilibria
48.3K Wyświetleń

16.6 : Obliczenia miareczkowania: mocny kwas - mocna zasada
Acid-base and Solubility Equilibria
28.9K Wyświetleń

16.7 : Obliczenia miareczkowania: słaby kwas - mocna zasada
Acid-base and Solubility Equilibria
43.7K Wyświetleń

16.8 : Wskaźniki
Acid-base and Solubility Equilibria
47.7K Wyświetleń

16.9 : Miareczkowanie kwasu poliprotonowego
Acid-base and Solubility Equilibria
95.6K Wyświetleń

16.10 : Równowaga rozpuszczalności
Acid-base and Solubility Equilibria
51.9K Wyświetleń

16.11 : Czynniki wpływające na rozpuszczalność
Acid-base and Solubility Equilibria
32.9K Wyświetleń

16.12 : Powstawanie jonów złożonych
Acid-base and Solubility Equilibria
23.1K Wyświetleń

16.13 : Wytrącanie jonów
Acid-base and Solubility Equilibria
27.4K Wyświetleń

16.14 : Analiza jakościowa
Acid-base and Solubility Equilibria
20.1K Wyświetleń

16.15 : Krzywe miareczkowania kwasowo-zasadowego
Acid-base and Solubility Equilibria
126.1K Wyświetleń
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone