Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Expanding Cytotoxic T Lymphocytes from Umbilical Cord Blood that Target Cytomegalovirus, Epstein-Barr Virus, and Adenovirus
W tym Artykule
Podsumowanie
Here we describe the first good manufacturing practice (GMP)-compliant method of producing virus-specific cytotoxic T lymphocytes (CTL) from umbilical cord blood, a source of predominantly naîve T cells.
Streszczenie
Virus infections after stem cell transplantation are among the most common causes of death, especially after cord blood (CB) transplantation (CBT) where the CB does not contain appreciable numbers of virus-experienced T cells which can protect the recipient from infection.1-4 We and others have shown that virus-specific CTL generated from seropositive donors and infused to the recipient are safe and protective.5-8 However, until recently, virus-specific T cells could not be generated from cord blood, likely due to the absence of virus-specific memory T cells.
In an effort to better mimic the in vivo priming conditions of naïve T cells, we established a method that used CB-derived dendritic cells (DC) transduced with an adenoviral vector (Ad5f35pp65) containing the immunodominant CMV antigen pp65, hence driving T cell specificity towards CMV and adenovirus.9 At initiation, we use these matured DCs as well as CB-derived T cells in the presence of the cytokines IL-7, IL-12, and IL-15.10 At the second stimulation we used EBV-transformed B cells, or EBV-LCL, which express both latent and lytic EBV antigens. Ad5f35pp65-transduced EBV-LCL are used to stimulate the T cells in the presence of IL-15 at the second stimulation. Subsequent stimulations use Ad5f35pp65-transduced EBV-LCL and IL-2.
From 50x106 CB mononuclear cells we are able to generate upwards of 150 x 106 virus-specific T cells that lyse antigen-pulsed targets and release cytokines in response to antigenic stimulation.11 These cells were manufactured in a GMP-compliant manner using only the 20% fraction of a fractionated cord blood unit and have been translated for clinical use.
Protokół
1. Mononuclear Cell Isolation (day 0)
- Insert spike of female luer adapter into outlet port of the 20% fraction of the cord blood unit, attach syringe and remove blood. Transfer thawed blood to 20 mL of warmed RPMI in 50 mL centrifuge tube. Rinse cord blood bag with 5 mL of RPMI and transfer to the same centrifuge tube.
- Centrifuge cells for 10 minutes at 400 x g. Aspirate supernatant.
- Resuspend cells in 20 mL of warm RPMI. Layer cells onto 15 mL of Lymphoprep in 50 mL centrifuge tube. Centrifuge 40 minutes @ 400 x g.
- Harvest the interface containing the mononuclear cells and wash in 20 mL of RPMI. Centrifuge for 10 minutes @ 450 x g.
- Aspirate supernatant and wash in 20 mL of RPMI. Count cells and spin for 5 minutes @ 400 x g.
- Aspirate supernatant and resuspend cells at 5 x 106 mononuclear cells/mL of Cellgenix DC media (serum free). Remove 1 mL for EBV-LCL generation and put into 15 mL centrifuge tube.
2. Dendritic Cell Generation (starting on day 0)
- Plate 2 mL of cells (are already at 5 x 106 cells/mL of DC media) per well into a 6-well plate. Leave in incubator for 1-2 hours.
- After 1-2 hours, wash off the non-adherent cells by washing 3-4 times with PBS. Collect the non-adherent cells and freeze. Add 2 mL/well of DC media containing 1000 U/mL of IL-4 and 800 U/mL GM-CSF.
- 3-4 days after DC initiation, replenish the IL-4 and GM-CSF by adding 100 μL/well of media containing a final concentration of 1000U/mL IL-4 and 800 U/mL GM-CSF.
- On day 5-6, harvest the DC by scraping the well with a transfer pipette. Count the big cells and centrifuge for 5 minutes @ 400 x g. Aspirate media and resuspend DC at 2x106 cells/mL of DC media. Add 0.5 mL/well of a 24-well plate.
- Transduce the DC using the Ad5f35pp65 vector at an MOI per cell of 10 infectious units. Add vector to each well in a 24-well plate. Incubate cells for 1.5 hours. After 1.5 hours, add 1.5 mL/well of DC media containing: 1000 U/mL IL-4, 800 U/mL GM-CSF, 10 ng/mL TNF-alpha, 1 μg/mL PGE-1, 100 ng/mL IL-6, and 10 ng/mL IL-1beta. These cytokines mature the DCs.
3. Generation of EBV-LCL (starting on day 0)
- Centrifuge the tube containing the 5 x 106 mononuclear cells. Add 200 μL of concentrated EBV B95.8 supernatant and 1.8 mL of complete media (RPMI + 10% FBS + 2mM L-glutamine) containing cyclosporine (1 μg/mL).
- Aliquot 100 μL of cells into 10 wells of a 96-well plate and 200 μL into 5 wells. Add 100 μL of complete media + cyclosporine to the wells containing only 100 μL of cells. Fill remaining wells with sterile water.
- Feed the LCL weekly and expand from a 96-well plate to a 24-well plate after ~2 weeks. After another week, transfer to a T25 flask and in the subsequent week transfer to a T75 flask. At this stage it is recommended that you freeze aliquots of LCL. It typically takes 1 month to generate sufficient numbers of LCL.
4. CTL Initiation – 7 days After Initiation of DCs
- Harvest DCs and irradiate at 30 Gy. Wash 4 x and count. Resuspend DC in T cell media containing human serum (45% RPMI, 45% CLICK's, 10% human serum, 2mM L-glutamine) @ 1 x 105 DCs/mL.
- Thaw non-adherent cells from step 2.2. Wash cells, and count. Resuspend at 2 x 106 non-adherent cells/mL in T cell media containing human serum. Add 10 ng/mL IL-7 and IL-12, and 5 ng/mL IL-15. Add 1 mL of cells per well in a 24-well plate. Add 1 mL of DC per well. Fill empty wells of 24-well plate with sterile water.
- 7 days after T cell initiation, feed and/or split the cells with T cell media containing human serum.
- 9-12 days after T cell initiation, freeze the T cells until the LCL are ready.
- Once LCL have expanded and are passaged in T75 flasks, transduce the LCL by centrifuging for 5 minutes @ 400 x g. Add the vector to the cell pellet at an MOI of 100 infectious units per cell. Incubate for 1.5 hours. After 1.5 hours, resuspend in complete media at 5 x 105 LCL/mL and add 2 mL per well in a 24-well plate.12
- After 2 days, harvest the LCL and irradiate at 40 Gy. Wash 4 x and count. Resuspend the LCL in T cell media containing human serum at 2.5 x 105 cells/mL. Add 1 mL of LCL per well of a 24-well plate. Also, add 5 x 106 LCL to a Grex40 culture device as well as 5 ng/mL IL-15.13
- Harvest the T cells. Centrifuge for 5 minutes @ 400 x g , aspirate the supernatant, and resuspend in T cell media containing human serum at 1 x 106 T cells/mL. Add 5 ng/mL IL-15 and add 1 mL/well of T cells (total of 1 x 106 T cells) to the LCL that are already in the 24-well plate. In the Grex40, bring the total volume of media up to 30 mL.
- On day 3-4 post-stimulation, if the cells are confluent then split them (in the plate) 1:1 and add fresh media containing 50 U/mL IL-2. If they are not confluent then simply aspirate ½ the media and replace with media containing 50 U/mL IL-2. In the Grex40, aspirate half the media, replace with fresh media, and add 50 U/mL IL-2.
- On day 7, repeat as in step 4.6 but use IL-2 on the day of the stimulation, not IL-15.
5. Representative Results
A schematic of the GMP-compliant FDA-approved manufacturing protocol is depicted in Figure 1. The process takes about 50 days. Typical methods of generating virus-specific T cells expand pre-existing memory T cells; however, cord blood lacks virus-experienced T cells and therefore we need to prime naïve T cells ex vivo. To do so, we use dendritic cells as well as the cytokines IL-7, IL-12, and IL-15, necessary for generating viral specificity.
After 3 stimulations, the yield should be over 100x106 cells. If sufficient cells are not available, additional stimulations can be performed until the desired number of CTLs are available. The majority of these cells should be CD3+ with a mixture of CD4+ and CD8+ T cells. There should be less than 15% NK cells (CD3-/CD56+) and less than 1% CD19+ B cells (Figure 2).
The expanded CTL should recognize the antigen pp65 from CMV, hexon and penton from adenovirus, as well as numerous EBV antigens that are expressed on EBV-LCL. When tested in an ELISPOT assay, CTL should secrete more IFN-? in response to these antigens than irrelevant antigens (Figure 3).
The CTL should also lyse viral peptide-pulsed targets such PHA blasts. In a 51Cr release assay, CTL should lyse LCL, CMVpp65-pulsed and Adenovirus hexon- and/or penton-pulsed targets but not targets pulsed with irrelevant peptides (Figure 4).
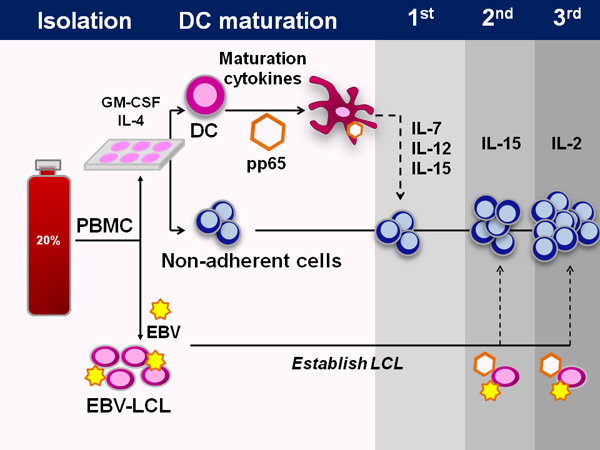
Figure 1. Generation of multi-virus-specific CTL from cord blood. Schematic showing the entire process of CTL manufacture from cord blood. First the cord blood mononuclear cells are isolated from the 20% fraction of the fractionated umbilical cord blood unit. With the exception of 5x106 cells that are saved for LCL generation, all the cells are then plated in dendritic cell media for 1-2 hours, at which point the non-adherent cells are harvested and frozen. The DC are then fed DC media containing IL-4 and GM-CSF. After 5 days of culture, the DC are matured and transduced with an adenoviral vector containing the immunodominant CMV antigen pp65. At initiation, these DC are combined with the non-adherent cells as well as the cytokines IL-7, IL-12, and IL-15. At subsequent stimulations, the same adenoviral vector is used to transduce EBV-LCL, which are used as the antigen presenting cells. IL-15 is used at the second stimulation and IL-2 thereafter.
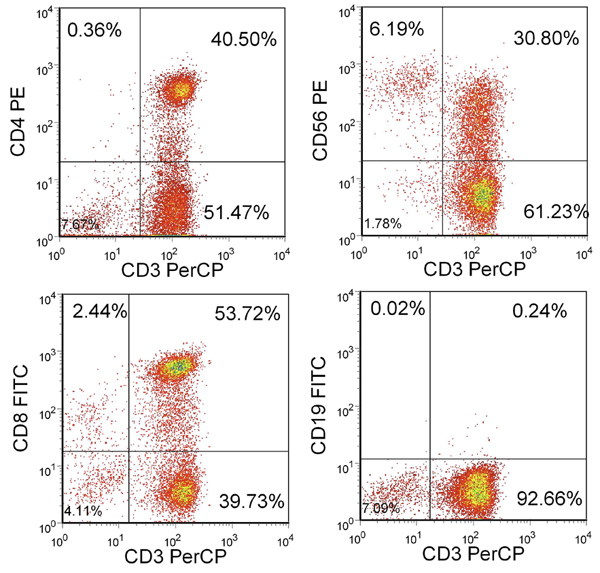
Figure 2. Phenotype of resulting T cells. Shown is the percentage of live cells included in the lymphocyte gate. CTL are CD3+ and CD4+ or CD8+ but largely CD3-/CD56- and CD19-.
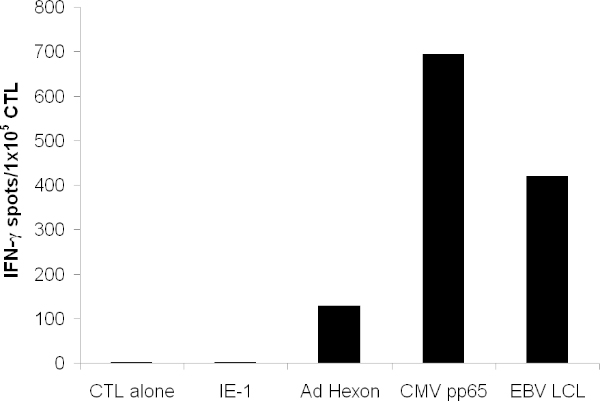
Figure 3. T cell functionality. T cell specificity was tested by IFN- γELISPOT. T cells were pulsed with overlapping peptides spanning the entire protein of hexon, penton, pp65, and the irrelevant antigen IE-1. CTL alone indicates media alone. Autologous LCL were irradiated and added at 1x105/well. Shown is the mean spot forming cell count of triplicate wells.
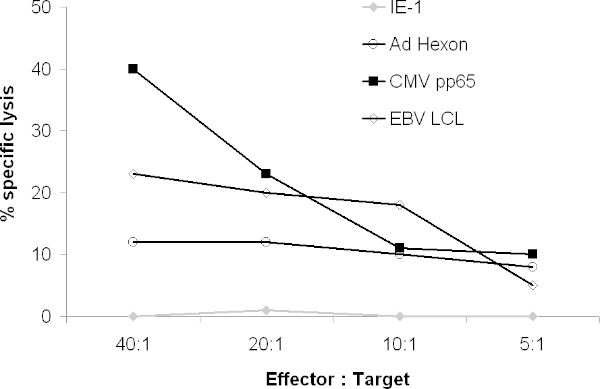
Figure 4. Cytolytic activity of CTL. The ability of the resulting CTL to lyse targets expressing viral antigens was tested in a 51Cr assay. 51Cr-labelled Autologous PHA blasts were pulsed with overlapping peptides spanning the entire antigen or 51Cr-labelled Autologous LCL were cocultured with CTL. After 4 hours, gamma release was counted on a gamma counter.
Dyskusje
Current strategies aimed at controlling viral infections after CBT can be effective, but they are associated with significant toxicities, are expensive, and do not confer long term protection against later infection. In fact, the use of some antiviral drugs may limit the expansion of virus-specific T cells that would otherwise be protective.14 Another option is the infusion of donor-derived virus-specific T cells. We and others have shown that such T cells are safe, efficacious, and cost-effective.15-17
Ujawnienia
The authors have nothing to disclose.
Podziękowania
This work was supported by a Dan L. Duncan collaborative research grant (C.M.B and E.J.S), the National Heart, Lung, and Blood Institute (US4HL081007), a Leukemia and Lymphoma Society Clinical Research Scholar award (C.M.B), and the National Cancer Institute (RO1 CA06150816; E.J.S).
Materiały
| Name | Company | Catalog Number | Comments |
| RPMI 1640 | Invitrogen | 21870-076 | |
| DC media | CellGenix | 20801-0500 | |
| EHAA (Click’s Medium) | Irvine Scientific | 9195 | |
| Human Serum | Gemini Bio Products | 100-110 | |
| Gas Permeable Cultureware18 | Wilson Wolf Manufacturing | 80040S | |
| IL-2 | Chiron (TCH Pharmacy) | ||
| IL-12 | National Cancer Institute | ||
| IL-15 | CellGenix | 1013-050 | |
| IL-7 | R&D Systems | AFL207 | |
| IL-1beta | R&D Systems | AFL201 | |
| IL-6 | CellGenix | 1004-050 | |
| GM-CSF | TCH Pharmacy | ||
| IL-4 | R&D Systems | AFL204 | |
| TNF-alpha | R&D Systems | AFL210 | |
| Ad5f35pp65 | BCM CAGT Vector Production Facility | ||
| Plasma transfer set with female luer adapter | Charter Medical | 89-550-66j | |
| Lymphoprep | Nycomed | 1114550 |
Odniesienia
- Kennedy-Nasser, A. A. Comparable outcome of alternative donor and matched sibling donor hematopoietic stem cell transplant for children with acute lymphoblastic leukemia in first or second remission using alemtuzumab in a myeloablative conditioning regimen. Biol. Blood Marrow Transplant. 14, 1245-1245 (2008).
- Hanley, P. J. Improving clinical outcomes using adoptively transferred immune cells from umbilical cord blood. Cytotherapy. 12, 713 (2010).
- Szabolcs, P., Cairo, M. S. Unrelated umbilical cord blood transplantation and immune reconstitution. Semin. Hematol. 47, 22 (2010).
- Canto, E., Rodriguez-Sanchez, J. L., Vidal, S. Distinctive response of naive lymphocytes from cord blood to primary activation via TCR. J. Leukoc. Biol. 74, 998-998 (2003).
- Leen, A. M. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat. Med. 12, 1160-1160 (2006).
- Riddell, S. R. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 257, 238 (1992).
- O'Reilly, R. J. Adoptive transfer of antigen-specific T-cells of donor type for immunotherapy of viral infections following allogeneic hematopoietic cell transplants. Immunol. Res. 38, 237-237 (2007).
- Peggs, K. S. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet. 362, 1375-1375 (2003).
- Sili, U. Large-scale expansion of dendritic cell-primed polyclonal human cytotoxic T-lymphocyte lines using lymphoblastoid cell lines for adoptive immunotherapy. J. Immunother. 26, 241 (2003).
- Bollard, C. M. Good manufacturing practice-grade cytotoxic T lymphocytes specific for latent membrane proteins (LMP)-1 and LMP2 for patients with Epstein-Barr virus-associated lymphoma. Cytotherapy. 13, 518 (2011).
- Hanley, P. J. Functionally active virus-specific T cells that target CMV, adenovirus, and EBV can be expanded from naive T-cell populations in cord blood and will target a range of viral epitopes. Blood. 114, 1958 (1958).
- Hanley, P. J. Expansion of T cells targeting multiple antigens of cytomegalovirus, Epstein-Barr virus and adenovirus to provide broad antiviral specificity after stem cell transplantation. Cytotherapy. , (2011).
- Gerdemann, U. Generation of Multivirus-specific T Cells to Prevent/treat Viral Infections after Allogeneic Hematopoietic Stem Cell Transplant. J. Vis. Exp. (51), e2736 (2011).
- Mori, T., Kato, J. Cytomegalovirus infection/disease after hematopoietic stem cell transplantation. Int. J. Hematol. 91, 588 (2010).
- Einsele, H. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood. 99, 3916 (2002).
- Bao, L. Expansion of cytomegalovirus pp65 and IE-1 specific cytotoxic T lymphocytes for cytomegalovirus-specific immunotherapy following allogeneic stem cell transplantation. Biol. Blood Marrow Transplant. 14, 1156 (2008).
- Shpall, E. J., Bollard, C. M., Brunstein, C. Novel cord blood transplant therapies. Biol. Blood Marrow Transplant. 17, S39-S45 (2011).
- Vera, J. F. Accelerated production of antigen-specific T cells for preclinical and clinical applications using gas-permeable rapid expansion cultureware (G-Rex. J. Immunother. 33, 305 (2010).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone