Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Collection Protocol for Human Pancreas
W tym Artykule
Podsumowanie
This video demonstrates a dissection procedure for processing human pancreas into multiple storage formats. Anatomical orientation is maintained throughout the pancreatic regions to allow definition of regional islet composition and density.
Streszczenie
This dissection and sampling procedure was developed for the Network for Pancreatic Organ Donors with Diabetes (nPOD) program to standardize preparation of pancreas recovered from cadaveric organ donors. The pancreas is divided into 3 main regions (head, body, tail) followed by serial transverse sections throughout the medial to lateral axis. Alternating sections are used for fixed paraffin and fresh frozen blocks and remnant samples are minced for snap frozen sample preparations, either with or without RNAse inhibitors, for DNA, RNA, or protein isolation. The overall goal of the pancreas dissection procedure is to sample the entire pancreas while maintaining anatomical orientation.
Endocrine cell heterogeneity in terms of islet composition, size, and numbers is reported for human islets compared to rodent islets 1. The majority of human islets from the pancreas head, body and tail regions are composed of insulin-containing β-cells followed by lower proportions of glucagon-containing α-cells and somatostatin-containing δ-cells. Pancreatic polypeptide-containing PP cells and ghrelin-containing epsilon cells are also present but in small numbers. In contrast, the uncinate region contains islets that are primarily composed of pancreatic polypeptide-containing PP cells 2. These regional islet variations arise from developmental differences. The pancreas develops from the ventral and dorsal pancreatic buds in the foregut and after rotation of the stomach and duodenum, the ventral lobe moves and fuses with the dorsal 3. The ventral lobe forms the posterior portion of the head including the uncinate process while the dorsal lobe gives rise to the rest of the organ. Regional pancreatic variation is also reported with the tail region having higher islet density compared to other regions and the dorsal lobe-derived components undergoing selective atrophy in type 1 diabetes4,5.
Additional organs and tissues are often recovered from the organ donors and include pancreatic lymph nodes, spleen and non-pancreatic lymph nodes. These samples are recovered with similar formats as for the pancreas with the addition of isolation of cryopreserved cells. When the proximal duodenum is included with the pancreas, duodenal mucosa may be collected for paraffin and frozen blocks and minced snap frozen preparations.
Protokół
1. Set-up
- Label cassettes for paraffin blocks manually with a pencil, or automatically with a cassette printer. Include the donor identifier (CaseID), sample type, aliquot number, and any additional unique identification (Case worksheet, Appendix 1).
- Label OCT molds as for cassettes with a permanent marker. Cut out 5-10 cm rectangles of aluminum foil.
- Print or manually label with permanent marker all vials and tubes. Include enough vials for minced snap frozen samples and tubes with RPMI media for fresh shipments. For the snap frozen vials with RNAlater, add 1 mL RNAlater to each cryovial.
- Set-up dissection boards in biosafety hood and counter tops with sterilized dissection instruments (forceps, scalpels, blades, gauze pads (4x4)) as well as cassettes and OCT molds arranged in order of use.
- Prepare a freezing bath by putting dry ice in a medium sized Styrofoam box (10x10 cm) to a depth of at least 3 cm and add isopentane until the dry ice is completely covered.
- Optional: Fill a small Dewar container with liquid nitrogen.
- If pancreas is needed for electron microscopy (EM), prepare TAAB fixative by adding 1.25 mL 16% paraformaldehyde, 1.25 mL 8% glutaraldehyde, and 7.5 mL 0.1M PBS. Prepare Lowicryl fixative by adding 2.5 mL 16% paraformaldehyde to 7.5 mL 0.1M PBS.
- Prepare solutions used for isolating cells. To 500 ml bottle of DMEF (with glutamine), follow aseptic techniques while adding the following:
- Fetal bovine serum (FBS): add 50mL, final 10%.
- Penicillin/Streptomycin (Pen/Strep, 10,000 U/mL / 10,000 ug/mL stock) - add 5 mL Pen/Strep stock to RPMI for final concentration of 100 U/mL / 100ug/mL. Also add 5 ml to a 500 ml bottle of DPBS when used for holding lymph nodes or spleen samples.
2. Personnel
- Procedure is optimally accomplished with three staff members however the procedure can be conducted with one well trained individual. Two individuals can generally complete the procedure in 90 minutes. Actual duration is dependent on numbers of tissues received and ease of finding pancreatic lymph nodes. Samples for cell isolations are held at 4 °C until completion of tissue dissection while serum samples are processed in parallel.
- Pathologist, pathology assistant, or equivalent- an experienced staff member leads the tissue handling and performs major dissections including division(s) of pancreas head and harvesting pancreatic lymph nodes. Initial efforts are directed towards pancreas followed by examination of peri-pancreatic fat for lymph nodes.
- Laboratory technologist or equivalent- staff member trained in tissue trimming whose primary responsibilities are to weigh pancreas and regions and trim tissues for fixed and frozen blocks and for snap frozen vials. Receives duodenum and spleen from pathology assistant and processes these organs while the pathology assistant is processing the pancreas.
- Laboratory assistant- staff member who assists the others including block and vial labeling, sample mincing for cryovials, and serum processing.
3. Pancreas Dissection
- Remove the duodenum and spleen and clean the pancreas of extraneous fat, vessels, and nerves using blunt and sharp dissection
- Place the cleaned pancreas on a dissecting board covered with paper towels. Dip a cotton-tipped applicator in blue ink and spread on the anterior surface of the pancreas. Blot off excess.
- Optional: Dip a new applicator in black ink and spread on the posterior surface of the pancreas. Return pancreas to normal orientation with anterior surface facing prosector.
- Cut the pancreas into 3 regions: head, body, and tail (Figure 1). Cut the junction between head and body then divide the remaining portion into ~equal portions for body and tail.
- Weigh each region of pancreas and record weights (Case workup sheet (Appendix 1)).
- Section (~0.5 cm) each region of the pancreas in a transverse "bread loaf" manner (Figure 1).
- Use the sections from both junctions between regions for minced samples. Depending on pancreas size, a sample from the body-tail junction region may be difficult to obtain in which case the number of paraffin blocks can be decreased to obtain minced samples or only samples from the pancreas head-body region can be obtained. Label cryovials according as head or body to denote proximal region at the junction.
- Mince pieces and divide evenly between cryovials (≥1 gram per vial). Rapidly freeze vials without RNALater in liquid nitrogen or in the dry ice - isopentane slurry then transfer to a -80 °C freezer.
- Keep vials with RNALater at room temperature for 30 minutes to allow for equilibration, remix and rapidly freeze in liquid nitrogen or in the dry ice-isopentane slurry then transfer to a -80 °C freezer.
- Remove alternating sections for paraffin and OCT blocks beginning with paraffin (Figure 1).
- Lean all transverse sections to the right.
- Place ~1.5 x 1.5 x 0.5 cm sections in the cassettes. If the transverse sections are too large, cut in half (Figure 2b). Label cassettes for blue anterior half as "A" and posterior half as "B".
- If pieces are still too large, cut each section perpendicular to the previous cut (Figure 2c). Label cassettes A-D in a clockwise manner. Trim pieces further as needed to fit within cassettes.
- Number blocks sequentially within region, starting with the most medial section.
- For paraffin blocks, position cassettes so the label is to the left and place tissue into the cassette, maintaining its orientation. Close cassette lid and transfer to a container with ~500 ml 10% neutral buffered formalin (NBF). Start timing fixation when the last cassette is placed in fixative.
- For OCT blocks, pour a thin layer of OCT media into the prelabeled mold then lay the tissue in the mold being sure to maintain orientation and cover tissue with OCT. Rapidly immerse the OCT molds in a dry ice - isopentane slurry for ~1 minute. Remove from slurry and place on dry ice for temporary storage or in -80 °C freezer for long term storage.
- Obtain ~0.5 x 0.5 cm slices from the head-body junction for electron microscopy (EM). Place one slice in the TAAB fixative and the other slice in the Lowicryl fixative. Store both types of fixative with samples at 4 °C for at least 48 hours and/or indefinitely until further processing for EM.
4. Spleen, Lymph Node Dissections and Duodenal Mucosa Dissections
- Isolate pancreatic lymph nodes (PLN) from fat and trim all connective tissue to the node capsule. Place in sterile cell culture dish containing DMEF. Depending on total numbers isolated, divide PLN into aliquots for fresh shipments, cryopreserved cell isolations, paraffin and/or OCT blocks, and vials. Mince large nodes into pieces no larger than 0.5 cm.
- Mince non-pancreatic lymph nodes and spleen into small (~0.5 cm) cubes. Place in fixative, OCT media, and or DMEM for fresh sample shipments or cryopreserved cell isolations according to amounts of starting materials.
- Open the duodenum and gently wipe the duodenal mucosa clean of mucous with a moistened gauze pad. Cut sections of the mucosa for paraffin and/or frozen OCT blocks and minced samples for vials, with and without RNAlater. Freeze as for other samples.
5. Wrap OCT blocks in pre-labeled aluminum foil and place OCT blocks and vials in boxes for long term storage at -80 °C.
6. Submit Fixed Samples for Paraffin Processing
- Fix tissues for 16 hours (range 20 ±4 hours) using an automatic paraffin processor or using manual timing. Process to paraffin blocks using an automatic processor (Appendix 2).
- Embed sections in the exact orientation as placed by the prosector with the cassette label to the left. Embed the duodenal mucosa with the cut surface down to allow examination of the mucosa perpendicular to the submucosa.
7. Clean dissection areas and disinfect. Wash dissection instruments and re-sterilize. Place remnant human tissues in formalin fixative for storage as biomedical waste. Discard biomedical waste according to local regulations within one month.
8. Update the Sample Inventory System
9. Representative Results
This procedure will process an intact human pancreas with representative sampling throughout the organ including demarcation of the three major regions, namely, head, body and tail within 2 hours. The uncinate region, found in the posterior head region, is included in the head dissection. The median pancreatic weight in organ donors without diabetes was 82.4 grams (52.7 - 139.0 grams). Pancreas regional weights were approximately equal (Figure 3).
This procedure can also allow for reconstruction of the entire pancreas using stained slides from sequential paraffin and OCT blocks. Multiple formats are feasible allowing for maximum utilization for current and future technologies. The current nPOD case worksheet is provided (Appendix 1) that is used to document recovered samples and subsequent processing by aliquot types and quantities.
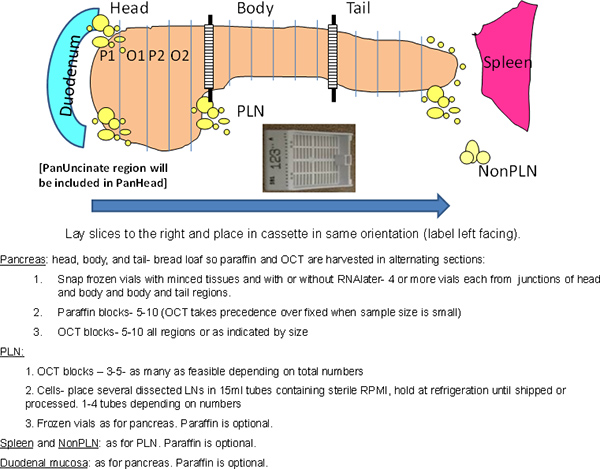
Figure 1. Overall scheme of the procedure. The entire pancreas is processed while maintaining anatomical orientations. The head region is longer in the superior-inferior axis due to the presence of the ventral pancreatic lobe in the posterior region that includes the uncinate region. Hatched area at junctions denote regions used for minced samples in cryovials. P, Paraffin; O, OCT.
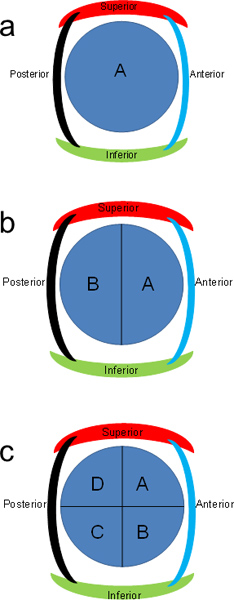
Figure 2. Lettering scheme for pancreas sections. Transverse pancreas slices can be further divided into halves or quarters and lettered in a clockwise manner.
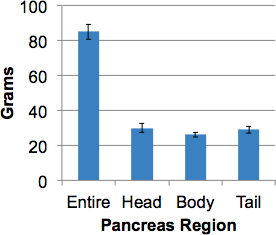
Figure 3. Pancreas weights from organ donors without diabetes. The intact pancreas from adult donors (>17 years old) was weighed then divided into regions and regional weights obtained. Data are means ± SEM (N=15).
Dyskusje
The goal of this procedure is to define a standard pancreas processing that can be harmonized across multiple collection sites and thus allow comparisons of results obtained by different groups. The dissection method is based on standard surgical pathology practices with additional steps for annotation of major pancreas regions followed by intra-regional subdivisions. Standardization of biospecimen processing methods is required to facilitate collection of high quality samples exemplified by the publication of best pract...
Ujawnienia
No conflicts of interest declared.
Podziękowania
The authors thank the donors' families and the Organ Procurement Organizations involved in this research and Maria Martino and Irina Kusmartseva for their expert assistance. This work was funded by the Juvenile Diabetes Research Foundation (M.C-T., M.A.) in support of the Network for Pancreatic Organ Donors with Diabetes.
Materiały
| Name | Company | Catalog Number | Comments |
| Dissection instruments including scalpels with 22 blades | Various | Various | |
| Centrifuge tubes (15 and 50 ml) | Fisher Scientific | 05-539-5, 430828 | Sterile |
| Cryotubes(1.8 ml) | VWR North America | 89004-300 | Sterile, bar codes optional |
| RNAlater | Ambion | AM7021 | |
| Surgical Pathology Ink | |||
| Cassettes | Mercedes Medical | CAS ECO109 | |
| 10% Neutral Buffered Formalin | Fisher Scientific | 23-245-685 | |
| OCT Freezing Media | TissueTek | 4583 | |
| Molds | Thermo Fisher Scientific, Inc. | 58952 | Various sizes |
| Isopentane | Fisher Scientific | O3551-4 | OCT chilling bath |
| Dry ice | OCT chilling bath | ||
| Liquid nitrogen in Dewar flask | Snap freezing cryovials | ||
| Cell culture dish (100mm) | Corning | 430167 | Sterile |
| Hyclone DMEF | Thermo Fisher Scientific, Inc. | SH30023.01 | Sterile, store refrigerated, glutamine included |
| Fetal Bovine Serum | Cellgro | 35-010-CV | Sterile, store frozen aliquots |
| Penicillin/Streptomycin/Antifungal | GIBCO, by Life Technologies | 15240-062 | Sterile, store frozen aliquots |
| Type 9 Paraffin | Richard-Allan Scientific | 8337 | |
| 1X D-PBS | GIBCO, by Life Technologies | 14190-144 | Sterile |
| 16% Paraformaldehyde | Electron Microscopy Sciences | 15710 | Make fresh, store refrigerated |
| 8% Glutaraldehyde | Electron Microscopy Sciences | 16019 | Make fresh, store refrigerated |
| Biosafety Level 2 hood | Various | ||
| Ultra-low freezer | Various | ||
| Sakura Cassette Printer | Sakura Finetek | ||
| Sakura VIP300 | Sakura Finetek | Automatic paraffin processor | |
| Paraffin Embedding Station | Thermo Fisher Scientific, Inc. | ||
| Table 1. Specific reagents and equipment. | |||
Odniesienia
- Brissova, M. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J. Histochem. Cytochem. 53, 1087-1097 (2005).
- Rahier, J. The pancreatic polypeptide cells in the human pancreas: the effects of age and diabetes. J. Clin. Endocrinol. Metab. 56, 441-444 (1983).
- Uchida, T., Takada, T., Ammori, B. J., Suda, K., Takahashi, T. Three-dimensional reconstruction of the ventral and dorsal pancreas: a new insight into anatomy and embryonic development. J. Hepatobiliary Pancreat. Surg. 6, 176-180 (1999).
- Wittingen, J., Frey, C. F. Islet concentration in the head, body, tail and uncinate process of the pancreas. Ann. Surg. 179, 412-414 (1974).
- Rahier, J., Goebbels, R., Henquin, J. Cellular composition of the human diabetic pancreas. Diabetologia. 24, 366-371 (1983).
- Vaught, J. An NCI perspective on creating sustainable biospecimen resources. J. Natl. Cancer Inst. Monogr. 42, 1-7 (2011).
- Gianani, R. Initial results of screening of nondiabetic organ donors for expression of islet autoantibodies. J. Clin. Endocrinol. Metab. 91, 1855-1861 (2006).
- Tauriainen, S., Salmela, K., Rantala, I., Knip, M., Hyöty, H. Collecting high-quality pancreatic tissue for experimental study from organ donors with signs of β-cell autoimmunity. Diabetes Metab. Res. Rev. 26, 585-592 (2010).
- In't Veld, P. Screening for insulitis in adult autoantibody-positive organ donors. Diabetes. 56, 2400-2404 (2007).
- Töns, H. A., Terpstra, O. T., Bouwman, E. Heterogeneity of human pancreata in perspective of the isolation of the islets of langerhans. Transplant Proc. 40, 367-369 (2008).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone