Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Three-Dimensionally Printed Microfluidic Cross-flow System for Ultrafiltration/Nanofiltration Membrane Performance Testing
W tym Artykule
Podsumowanie
Design and fabrication of a three-dimensionally (3-D) printed microfluidic cross-flow filtration system is demonstrated. The system is used to test performance and observe fouling of ultrafiltration and nanofiltration (thin film composite) membranes.
Streszczenie
Minimization and management of membrane fouling is a formidable challenge in diverse industrial processes and other practices that utilize membrane technology. Understanding the fouling process could lead to optimization and higher efficiency of membrane based filtration. Here we show the design and fabrication of an automated three-dimensionally (3-D) printed microfluidic cross-flow filtration system that can test up to 4 membranes in parallel. The microfluidic cells were printed using multi-material photopolymer 3-D printing technology, which used a transparent hard polymer for the microfluidic cell body and incorporated a thin rubber-like polymer layer, which prevents leakages during operation. The performance of ultrafiltration (UF), and nanofiltration (NF) membranes were tested and membrane fouling could be observed with a model foulant bovine serum albumin (BSA). Feed solutions containing BSA showed flux decline of the membrane. This protocol may be extended to measure fouling or biofouling with many other organic, inorganic or microbial containing solutions. The microfluidic design is especially advantageous for testing materials that are costly or only available in small quantities, for example polysaccharides, proteins, or lipids due to the small surface area of the membrane being tested. This modular system may also be easily expanded for high throughput testing of membranes.
Wprowadzenie
Membrane technology is integral to industrial and other processes requiring the separation of solutes from a bulk solution, however, membrane fouling is a major ongoing challenge.1 Common examples where membrane fouling occurs include the use of ultrafiltration membranes for the size based separation of wastewater,2 and thin film composite membranes for the separation of ions and larger solutes from brackish or seawater.3 Characteristic indications of fouling include an increase in transmembrane pressure and a decline in flux. This decreases the productivity of the membrane and shortens its lifetime due to chemical or other cleaning protocols. Therefore membrane performance is a good indicator to assess fouling and to understand the mechanisms and effects of fouling, biofouling and biofilm formation on membranes. Also, performance assessment is important in the design or modification of new membranes.
Interest in the use of membranes in microfluidic devices has been growing over the last decade.4 Recently, we studied the effect of microbial components lipopolysaccharide, and glycosphingolipid on fouling the surface of a nanofiltration membrane, and the subsequent susceptibility of the conditioned surface to microbial attachment.5 A microfluidic cross-flow device was used to assess the performance of nanofiltration membranes. This allowed the use of special non-commercial lipid components only available in small quantities for membrane surface fouling because the membrane surface area was small. The system size allowed efficient use of membrane materials and low volumes of solutions. In this protocol, we describe the design and fabrication of the microfluidic device for membrane performance testing, and outline the incorporation of the device into a pressure flow system. Demonstration of the device is shown by testing the performance of ultrafiltration membranes and nanofiltration membranes using a model foulant, BSA.6,7
Protokół
1. Design and Fabrication of the Microfluidic Test System
- Design microfluidic device as two separate parts: a top part and bottom part (Figure 1) in a CAD program.
- Start making the bottom part by using the rectangle tool to draw a 40 mm by 60 mm rectangle.
- At one corner with the circle tool create a 6.2 mm diameter circle centered 10 mm from edges. With the linear pattern tool replicate the holes across the rectangle with 20 mm spacing for a total of 6 holes.
- Using the fillet tool fillet the rectangles with a radius of 1 mm.
- Extrude the part 10 mm with the extrude tool.
- In the center of the top face, with the rectangle tool create a rectangle 30 mm by 1 mm and with the extrude cut tool cut 0.2 mm for the flow channel.
- Using the circle tool make a 1 mm diameter circle at the end of the flow channel. Then with the line tool construct a path connecting the circle to the nearest 40 mm by 10 mm face, including a 4 mm radius made with the fillet tool. Make a cut along this path with the swept cut tool.
- With the circle tool create a 3.9 mm diameter circle at the center of the flow path and cut 8 mm with the extrude cut tool to allow for fittings.
- Repeat steps 1.7 and 1.8 for the opposite side of the flow channel.
- With the top part repeat steps 1.2-1.5. Then in center of the top face create a permeate channel using the rectangle tool to create a rectangle 30 mm by 1 mm and cut 0.5 mm using the extrude cut tool.
- Use the circle tool to make a 1 mm circle centered in the permeate channel 5 mm from an end. With the line tool construct a path connecting the circle to one of the 1 cm by 6 cm faces, including a 4 mm radius made with the fillet tool. Make a cut along the path with the swept cut tool.
- With the circle tool create additional 3.9 mm diameter circle with its center on the permeate path and cut 8 mm with the extrude cut tool.
- At the parts top 40 mm edges, with the rectangle tool, create rectangles 40 mm by 5 mm adding 4 mm radii with the fillet tool. Use the extrude tool to extrude 3 mm downward for handles.
- Print parts with a multi-material photopolymer 3-D printer using a hard transparent polymer, including 0.05 mm overcoating with a soft rubbery polymer on the face of each part that contains the channel. Use manufacturer’s standard protocol, calibration and settings.
- Tap threads (M5) into feed, retentate and permeate orifices. Use plumber’s tape to connect 1/8” fittings to the feed and retentate and 1/16” fittings to the permeate.
- Connect microfluidic devices to pump, valves, pressure transducer and backpressure regulator with 1/8” tubing (Figure 2).
- Connect 0.45 μm filters to inlet tubes.
- Discharge permeate to flow-meter and beakers on balances with 1/16” tubing.
- Attach continuous rotation servo to backpressure regulator with screws and standard servo to 3-way valve with tie-wire.
- Connect servos and power supply to servo shield.
- Connect pressure transducer, switches and servo shield to microcontroller.
- Connect microcontroller, balances, flow-meter and pump to a PC for data logging and system control.
- Configure balances to print data to their serial port.
2. Prepare Membranes to Be Tested
- Cut membranes to 40 mm x 8 mm.
- Soak membranes in ultrapure water (3 x 10 min) with sonication.
- Then soak the membranes in 50/50 ultrapure water/ethanol for 1 hr.
- Rinse the membranes with ultrapure water and store in ultrapure water at 4 °C.8
3. Prepare Solutions to Be Tested with Nanofiltration Membranes
- Add 500 ml of ultrapure water to an Erlenmeyer flask. Then add 0.04 g of BSA and 0.29 g of NaCl.
- Add 500 ml of ultrapure water to a separate Erlenmeyer flask. Then add 0.6 g of MgSO4.
- Add 500 ml of ultrapure water to a third Erlenmeyer flask. Then add 0.29 g of NaCl.
- Insert stir bars into each flask and place flasks on stir plates. Mix for 5 min at 500 rpm.
4. Perform a Nanofiltration Fouling Experiment
Note: Perform the experiment at RT (ca. 24 °C). First configure the system for measuring a single membrane by closing valves to flow cells not connected to the flow-meter.
- Insert one pump inlet tube into the ultrapure water reservoir and the other inlet tube into the MgSO4 solution (Figure 2).
- Use a syringe to draw water and MgSO4 solution through tubing so as to remove all air bubbles in the system.
- Insert a nanofiltration membrane on the bottom part of the flow cell, with the active side towards the feed channel, and place on the top part of the flow cell.
- Fasten nuts by hand and then tighten evenly with a wrench so as to minimize leakage.
- Select the ultrapure water with the reservoir selector switch.
- Set pump flow rate to 2 ml/min and start the pump.
- Adjust pressure regulator to 4 bar.
- Set experimental parameters to switch reservoirs every 45 min starting with the water reservoir.
- Set reservoir switch to auto, and start experiment.
- At 60 min collect MgSO4 permeate in a tube for next 30 min.
- At 91 min replace MgSO4 flask with flask containing the solution of BSA and NaCl.
- Quickly stop pump and use a syringe to draw BSA solution through the inlet tube to remove MgSO4 leftover in tubing. Then start pump again.
- At 150 min collect BSA permeate in a tube for next 30 min.
- After 225 min, shut down the system and remove nanofiltration membrane from the flow cell.
- Using a syringe, flush out test solution inlet tube with ultrapure water.
- Repeat steps 4.1-4.15 for each additional membrane tested.
- For NaCl only tests, repeat steps 4.1-4.10, and 4.14-4.16 replacing MgSO4 solution with NaCl solution and ending the experiment after 90 min instead of 225 min.
5. Calculate Salt Rejection of Nanofiltration Membranes
- Rinse electrodes of the potentiostat test cell with ultrapure water.
- With a pipette, deposit 5 μl of MgSO4 solution onto the test cell electrodes.
- Record resistance of the solution.
- Repeat steps 5.1-5.3 four more times and calculate the average value.
- Repeat steps 5.1-5.4 for the NaCl and BSA/NaCl solutions as well as for each permeate solution collected.
- Calculate salt rejection with Equation 1:
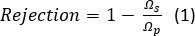
where Ωs is the resistance of the test solution and Ωp is the resistance of the permeate. The resistance is inversely proportional to conductivity of a solution, which directly correlates to salt concentration.
6. Prepare Solution to Be Tested with Ultrafiltration Membranes
- Add 1 L of ultrapure water to a 4 L beaker. Then add 0.32 g of BSA.
- Insert stir bar into beaker and place on a stir plate. Mix for 5 min at 500 rpm.
- Add additional 3 L of ultrapure water to beaker and mix again for 5 min at 500 rpm.
7. Perform an Ultrafiltration Fouling Experiment
Note: Perform an experiment at RT (ca. 24 °C). First configure the system to measure 4 membranes in parallel by opening all valves to flow cells.
- Place one pump inlet tube into the ultrapure water reservoir and other inlet tube into the BSA solution (Figure 2).
- Use a syringe to draw the water and the BSA solution through the tubing so as to remove all air bubbles in the system.
- Insert ultrafiltration membranes on the bottom part of the flow cells, with the active sides towards the feed channels, and close the cells with the top halves of the microfluidic device.
- Fasten nuts by hand, then tighten evenly with a wrench. Improper tightening may lead to water leakage.
- Select ultrapure water with reservoir switch.
- Set pump flow rate to 8 ml/min and start the pump.
- Adjust pressure regulator to 0.4 bar.
- Monitor flux values of membranes with data acquisition software according to manufacturer’s protocol.
- Adjust pressure regulator until average flux is 200 LMH ± 10%.
- Replace individual membrane if flux is not 200 LMH ± 20%.
- Enter experimental run parameters. First select the ultrapure water reservoir for 60 min with a constant flux of 200 ± 20 LMH. Then, select the BSA reservoir for 420 min with manual control of the pressure regulator. Finally, select the ultrapure water reservoir for 15 min with manual control of the pressure regulator to the flush system at the end of experiment.
- Set reservoir switch to auto, and start experiment.
- After run completion, shut system down and remove membranes from flow cells.
- With a syringe, flush pump inlet tube with ultrapure water.
Wyniki
The microfluidic flow cells were designed using a CAD program and printed using a multi-material photopolymer three-dimensional (3-D) printer. This cell was designed in two parts, so that membranes could be easily inserted and removed from the device (Figure 1). Each part was 1 cm thick, printed from a hard, clear polymer for structural integrity, and the sides facing the membrane were overcoated with a very thin 50 µm layer of rubber-like polymer. The overcoating was performed to provide the cell w...
Dyskusje
This protocol describes the design of a three-dimensionally printed microfluidic cross-flow device for testing of nanofiltration and ultrafiltration membranes. Recently, we have shown the success of a variation of this protocol with nanofiltration membrane conditioning and fouling with glycosphingolipids and lipopolysaccharides and membrane performance differences with subsequent bacterial culture injection.5 Future applications employing this technique could be used to evaluate membrane performance changes wi...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
The authors thank Stratasys (Rehovot, Israel) for three-dimensional printing of the device. We are grateful to Microdyne-Nadir (Germany) for the membrane samples. This research was supported by The Israel Science Foundation (Grant 1474-13) to C.J.A.
Materiały
| Name | Company | Catalog Number | Comments |
| BSA | SIGMA-ALDRICH | A6003 | |
| NaCl | DAEJUNG | 7548-4100 | |
| MgSO4 | EMSURE | 1058861000 | |
| NF Membrane | Filmtec | NF200 | |
| 30 kDa UF Membrane | MICRODYN NADIR | UH030 | |
| 50 kDa UF Membrane | MICRODYN NADIR | UH050 | |
| Pressure Transducer | Midas | 43006711 | |
| Ball Valves | AV-RF | Q91SA-PN6.4 | |
| 3-way Valve | iLife Medical Devices | 902.071 | |
| Pressure Regulator | Swagelok | KCB1G0A2A5P20000 | |
| Flow-meter | Bronkhorst | L01-AGD-99-0-70S | |
| Balances | MRC | BBA-1200 | |
| Pump | Cole-Parmer | EW-00354-JI | |
| 1/8" Tubing | Cole-Parmer | EW-06605-27 | |
| 1/16" Tubing | Cole-Parmer | EW-06407-41 | |
| 1/16" Fittings | Cole-Parmer | EW-30486-70 | |
| 1/8" Fittings | Kiowa | QSM-B-M5-3-20 | |
| Microcontroller | Adafruit | 50 | Arduino UNO R3 |
| Continuous Rotation Servo | Adafruit | 154 | |
| Standard Servo | Adafruit | 1142 | |
| Power Supply | Adafruit | 658 | |
| Servo Shield | SainSmart | 20-011-905 | |
| Switches | Parts Express | 060-376 | |
| 0.45 Micron Filters | EMD Millipore | SLHV033RS | |
| Potentiostat | Gamry | PCI4 | |
| Sonicator | MRC | DC-150H | |
| Connex 3D Printer | Stratasys | Objet Connex | |
| Veroclear | Stratasys | RGD810 | transparent polymer for printing flow cell |
| Tangoblack-plus | Stratasys | FLX980 | soft rubbery polymer for gasket layers on flow cell |
Odniesienia
- Guo, W., Ngo, H. -. H., Li, J. A mini-review on membrane fouling. Bioresource technol. 122, 27-34 (2012).
- Fane, A. G., Fell, C. J. D. A review of fouling and fouling control in ultrafiltration. Desalination. 62, 117-136 (1987).
- Tang, C. Y., Chong, T. H., Fane, A. G. Colloidal interactions and fouling of NF and RO membranes: a review. Adv. colloid interfac. 164 (1-2), 126-143 (2011).
- De Jong, J., Lammertink, R. G. H., Wessling, M. Membranes and microfluidics: a review. Lab on a chip. 6 (9), 1125-1139 (2006).
- Haas, R., Gutman, J., et al. Glycosphingolipids Enhance Bacterial Attachment and Fouling of Nanofiltration Membranes. Environ. Sci. Technol. Lett. 2, (2015).
- Nabe, A. Surface modification of polysulfone ultrafiltration membranes and fouling by BSA solutions. J. Membr. Sci. 133 (1), 57-72 (1997).
- Ang, W., Elimelech, M. Protein (BSA) fouling of reverse osmosis membranes: Implications for wastewater reclamation. J. Membr. Sci. 296 (1-2), 83-92 (2007).
- Bernstein, R., Belfer, S., Freger, V. Surface modification of dense membranes using radical graft polymerization enhanced by monomer filtration. Langmuir. 26 (14), 12358-12365 (2010).
- Kaufman, Y., Kasher, R., Lammertink, R. G. H., Freger, V. Microfluidic NF/RO separation: Cell design, performance and application. J. Membr. Sci. 396, 67-73 (2012).
- Kaufman, Y., et al. Towards supported bolaamphiphile membranes for water filtration: Roles of lipid and substrate. J. Membr. Sci. 457, 50-61 (2014).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone