Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Meiotic Spindle Assessment in Mouse Oocytes by siRNA-mediated Silencing
W tym Artykule
Podsumowanie
Here, we present a protocol for specific siRNA-mediated mRNA depletion followed by immunofluorescence analysis to evaluate meiotic spindle assembly and organization in mouse oocytes. This protocol is suitable for in vitro depletion of transcripts and functional assessment of different spindle and/or MTOC-associated factors in oocytes.
Streszczenie
Errors in chromosome segregation during meiotic division in gametes can lead to aneuploidy that is subsequently transmitted to the embryo upon fertilization. The resulting aneuploidy in developing embryos is recognized as a major cause of pregnancy loss and congenital birth defects such as Down’s syndrome. Accurate chromosome segregation is critically dependent on the formation of the microtubule spindle apparatus, yet this process remains poorly understood in mammalian oocytes. Intriguingly, meiotic spindle assembly differs from mitosis and is regulated, at least in part, by unique microtubule organizing centers (MTOCs). Assessment of MTOC-associated proteins can provide valuable insight into the regulatory mechanisms that govern meiotic spindle formation and organization. Here, we describe methods to isolate mouse oocytes and deplete MTOC-associated proteins using a siRNA-mediated approach to test function. In addition, we describe oocyte fixation and immunofluorescence analysis conditions to evaluate meiotic spindle formation and organization.
Wprowadzenie
Meiosis is a unique division process that occurs in gametes (oocytes and sperm) and involves two successive divisions without intervening DNA synthesis to segregate homologous chromosomes and sister chromatids during meiosis-I and meiosis-II, respectively1. Errors in chromosome segregation during meiotic division in oocytes can result in aneuploidy, which is inherited by the embryo during fertilization. Notably, the incidence of aneuploidy in developing embryos increases with advancing maternal age and is a major cause of congenital birth defects as well as pregnancy loss in women1,2, thus, underscoring an important need to understand the molecular basis of aneuploidy during meiotic division.
During cell division, chromosome segregation is crucially dependent on assembly of the microtubule spindle apparatus and establishment of stable chromosome-microtubule interactions for correct attachment to opposite spindle poles. Importantly, meiotic spindle formation in mammalian oocytes differs from mitosis in somatic cells, and is regulated by unique microtubule-organizing centers (MTOCs) that lack centrioles3,4. Essential proteins necessary for microtubule nucleation and organization localize to oocyte MTOCs, including γ-tubulin that catalyzes microtubule assembly. In addition, pericentrin functions as an essential scaffolding protein, which binds and anchors γ-tubulin as well as other factors at MTOCs5. Notably, our studies demonstrate that depletion of key MTOC-associated proteins disrupts meiotic spindle organization and leads to chromosome segregation errors in oocytes, which are not fully resolved by the spindle assembly checkpoint (SAC)6,7. Therefore, defects in spindle stability, that do not trigger meiotic arrest, pose a significant risk in contributing to aneuploidy. Despite their essential role in spindle assembly and organization, oocyte MTOC protein composition and function remains poorly understood.
Testing the function of specific target proteins in mammalian oocytes is challenging, as the cells become transcriptionally quiescent shortly before the resumption of meiosis8,9. Hence, pre-ovulatory oocytes rely on maternal mRNA stores to resume meiosis and support meiotic division as well as the first cleavage divisions after fertilization10,11. The efficacy of RNA interference (RNAi) mediated degradation of mRNA transcripts in mammalian oocytes is well established and maternal RNAs recruited for translation during meiotic maturation are particularly amenable to siRNA targeting 12-14. Therefore, microinjection of short interfering RNAs (siRNAs) into oocytes provides a valuable approach to deplete target mRNAs for functional testing.
Here, we describe methods for the isolation of mouse oocytes and siRNA-mediated depletion of specific transcripts to test the function of an essential MTOC-associated protein, pericentrin. In addition, we describe immunofluorescence analysis conditions to evaluate meiotic spindle formation in oocytes.
Protokół
This protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Georgia.
1. Preparations
- For oocyte culture, purchase or freshly prepare Minimal Essential Medium (MEM) and supplement with 3 mg/ml bovine serum albumin (BSA) as outlined in Table 1. Place a polystyrene bottle on a loading balance (tare to zero). Add all reagents, except BSA, in order and bring up the final volume with MQ-water by weight to a total of 250 g. Then add the BSA, allow to dissolve and filter sterilize.
Note: The described MEM medium requires equilibration and cell incubation with a medical gas mixture of 5% CO2, 5% O2 and 90% N2. However, other media (e.g., CZB) can be used that allow for cell incubation in 5% CO2 under atmospheric conditions. - For oocyte microinjection, purchase or prepare M2 medium.
- Prepare pregnant mare’s serum gonadotropin (PMSG) to a concentration of 5 IU/100 µl.
- Purchase and reconstitute siRNA stocks to a working concentration of 1 µM.
2. Mouse Oocyte Collection
- DAY 1: To stimulate ovarian follicle development and increase the number of pre-ovulatory follicles, administer 5 IU of PMSG intraperitoneally to female mice 48 hr prior to oocyte collection.
- DAY 3: For oocyte collection and culture, set up 35 mm culture dishes with 3 ml of MEM/BSA supplemented with 1 µg/ml milrinone. This phosphodiesterase inhibitor maintains the oocytes in prophase-I arrest and prevents germinal vesicle breakdown (GVBD). Equilibrate the culture dishes in a medical gas mixture for at least 15 min.
Note: Milrinone supplemented media is required throughout oocyte collection, as well as oocyte microinjection and the 24 hr culture hold period post siRNA. - Retrieve ovaries from the female mice using established laboratory practices15 and transfer to a new culture dish with pre-warmed and equilibrated MEM/BSA/milrinone.
- Place the culture dish on the stage of a stereomicroscope for oocyte collection. Release cumulus-oocyte-complexes (COC’s) by manually puncturing the antral follicles with 27 G needles. Fix one ovary to the bottom of the culture dish, with a 27 G needle attached to a 1 ml syringe, and use a second needle to puncture all large follicles. Repeat for each ovary.
- Using standard mouth pipetting procedures or other means of oocyte aspiration, collect all oocytes that are surrounded by 2 or more layers of compact cumulus cells. Transfer the COC’s to a new dish and incubate at 37 °C for 1 hr.
- Carefully remove the surrounding cumulus cells by repeated gentle pipetting with a 1 ml pipette set to an aspiration volume of 750 µl. Repeat the pipetting step about 12 times and allow the oocytes to recover for 5 min. Continue in this manner until all or most of the cumulus cells have been removed. Transfer the denuded oocytes to another culture dish and allow to equilibrate at 37 °C for 15 min.
- Allocate the denuded oocytes into three experimental groups to be used for: (i) microinjection of specific siRNAs against the target of interest, (ii) microinjection of unspecific (control) siRNAs, and (iii) a non-injected control group to account for culture conditions.
3. Oocyte Microinjection
- Set up culture dishes with 3 ml M2 media supplemented with milrinone (1 µg/ml) for oocyte microinjection. Prepare culture dishes containing MEM/BSA/milrinone for washing and subsequent culture of injected oocytes.
Note: M2 medium contains HEPES buffer and requires pre-warming at 37 °C for 15 min under atmospheric conditions. MEM requires pre-warming and equilibration using medical gas as described above. - Prepare the microinjection system. Load the injection needle with 5 µl of 1 µM specific siRNA solution. Secure the holding pipette and injection needle to micromanipulators of the microinjection system and set up the Injection Unit with calibrated volume and pressure.
- Place a 200 µl micro-drop of M2 media on the inside of the lid of a 3 cm culture dish and add an oocyte for set up and alignment of the microinjection system.
- Using the oocyte as a guide, adjust the positions of the holding and injection needles. Apply negative pressure to the holding pipette to gently secure the oocyte and adjust the position of the injection needle to the widest diameter of the cell. Adjust settings to allow microinjection of approximately 10 pl of the siRNA solution anywhere into the oocyte cytoplasm.
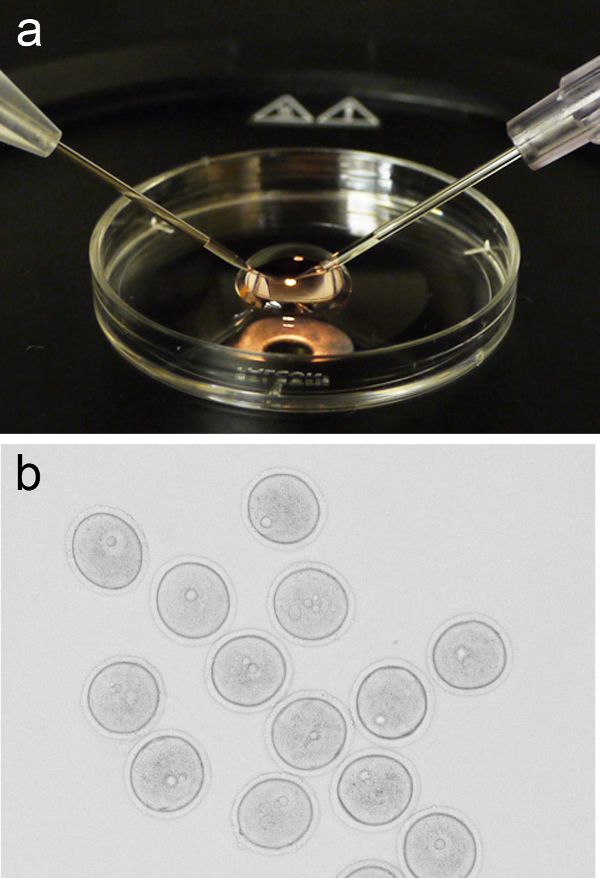
Figure 1. Oocyte microinjection set up. (A) Representative image of a 200 µl micro-drop of M2 media placed on the lid from a 3 cm culture dish, with the holding pipette and injection needle positioned on the left and right, respectively. (B) Group of prophase-I arrested oocytes (GV-stage) prior to microinjection. Please click here to view a larger version of this figure.
- Return the lid to the stereomicroscope stage and add a 200 µl micro-drop of fresh M2 media. Add a group of oocytes (~10) to the micro-drop by mouth pipetting, or alternative methods, then return the lid with micro-drop to the microinjection stage.
- Proceed to microinject the individual oocytes. Secure one oocyte with the holding pipette, slowly insert the tip of the injection needle into the cytoplasm and expel the injection volume of siRNA solution. Carefully retract the injection needle and move the injected oocyte to a position at the bottom of the micro-drop. Move unsuccessfully injected oocytes to a position at the top of the micro-drop.
- Repeat the microinjection procedure with each oocyte. To maintain optimal oocyte viability, microinject only a small number of oocytes at a time. Ensure that this process does not take more than a few minutes to prevent temperature and pH changes in the micro-drop.
- Aspirate successfully injected oocytes or carefully move the lid with the micro-drop of injected oocytes to the stereomicroscope. Transfer the injected viable oocytes by mouth pipetting or alternative methods, to MEM/BSA/milrinone medium to wash and equilibrate at 37 °C.
- Using another group of oocytes, repeat the microinjection process with a new injection needle loaded with non-specific siRNAs for the control group. Wash the oocytes in MEM/BSA/ milrinone and transfer to a separate culture dish.
- Culture all groups of oocytes for 24 hr in MEM/BSA/milrinone at 37 °C under an atmosphere of medical gas.
Note: The ‘milrinone block’ culture period is needed for efficient target mRNA/protein depletion.
4. Oocyte Culture for Meiotic Maturation
- DAY 4: For each experimental group of oocytes set-up 4 culture dishes (35 mm) with 3 ml MEM/BSA. Supplement one media dish per group with 10% (high-quality) fetal bovine serum (FBS) to use for oocyte maturation. Allow all culture dishes to equilibrate at 37 °C with medical gas mixture.
- To release oocytes from ‘milrinone block’, sequentially wash the oocytes three times in dishes containing MEM/BSA and then transfer the oocytes to the maturation dish containing media with 10% FBS. Culture all oocyte groups for 17 hr at 37 °C, to enable resumption and progression of meiosis.
- DAY 5: Prepare solutions for oocyte fixation, including: (i) 4% paraformaldehyde (PFA) in PEM buffer (100 mM PIPES [pH 6.9], 1 mM MgCl2, 1 mM EGTA) with 0.5% Triton-X 100, and (ii) PBS supplemented with 5% FBS that will be used to wash and block the oocytes.
Note: These solutions require pre-warming to 37 °C prior to oocyte fixation. - For oocyte fixation (after the 17 hr culture), swiftly transfer each experimental group of oocytes by mouth pipetting or alternative methods into individual wells (of a 4-well dish) containing 750 µl of pre-warmed 4% PFA solution and incubate at 37 °C for 1 hr. Subsequently wash each group of oocytes 3 times (15 min each) in 750 µl of pre-warmed PBS containing 5% FBS.
- Block oocytes in 200 µl PBS/5% FBS O/N at 4 °C to minimize unspecific antibody binding.
5. Immunofluorescence Analysis
- DAY 6:
Note: Immunostaining is done in a multi-well plate and the oocytes are serially transferred to sequential wells, containing respective antibody and wash solutions. Either 48 or 96-well plates can be used, simply adjust the solution volume based on well size. For 96-well plates use 200 µl per well. Oocytes are transferred to the different solutions in the following order: primary antibody solution - 3 wash wells - secondary antibody solution - 3 wash wells. To limit light exposure the plate can be covered with foil.
Prepare fresh PBS/5% FBS and use this solution to prepare antibody dilutions (e.g., rabbit anti-pericentrin (1/1,000), mouse anti-tubulin (1/1,000)). - Transfer the fixed oocytes of each experimental group to individual wells containing 200 µl (or 100 µl, if less volume is desired) of the primary antibody solution and cover the well with parafilm. Incubate according to optimal antibody-specific conditions (e.g., 37 °C for 1 hr, or 4 °C O/N).
Note: Primary antibody dilution as well as specific incubation time and temperature require testing to determine the optimal conditions for different antibodies used. - After incubation with the primary antibody, wash the oocytes three times in PBS/5% FBS (10-15 min each).
- Transfer the oocytes into the solution containing fluorescently-conjugated secondary antibodies (e.g., 1/1,000 dilution of anti-rabbit and anti-mouse antibodies conjugated with fluorochromes of different wavelengths). Incubate for 1 hr at 37 °C.
- Wash the oocytes three times in PBS/5% FBS (10 – 15 min each).
- After the final wash step, transfer the oocytes onto a clean glass slide and gently aspirate any excess wash solution. This will immobilize the oocytes on the glass surface. Add 8 µl of mounting media (containing DAPI) and carefully overlay the mounting media with a 22 mm x 22 mm cover slip. Lower the coverslip slowly to avoid trapping air bubbles and/or damaging the oocytes.
Note: Alternatively, to maintain the 3-dimentional properties of the oocyte for confocal microscopy analysis, add a small volume of 100 µm glass beads (mixed with petroleum jelly) to the corners of the cover slip prior to mounting on the slide. - Store slides at 4 °C, or proceed to the assessment of meiotic progression as well as the analysis of expression levels and subcellular protein localization using a fluorescence microscope equipped with the necessary filters to match the secondary antibodies used.
Wyniki
Microinjection of siRNAs provides an effective approach for mRNA degradation and subsequent protein depletion in oocytes, which enables efficient and highly specific functional testing of different target factors in vitro. Subsequently, immunofluorescence is used for specific phenotype analysis as well as to validate protein depletion in siRNA-injected oocytes. In the current example, fluorescent labeling of individual oocytes with DAPI together with anti-tubulin and anti-pericentrin antibodies enabled: (i) conf...
Dyskusje
While there are multiple methods for exogenous nucleic acid transfer into somatic cells, such as electroporation and transfection, microinjection is the optimal method for delivery of RNA molecules into transcriptionally quiescent mouse oocytes. The current protocol provides an effective approach for in vitro depletion of specific mRNAs that enable the functional testing of different spindle and/or MTOC-associated factors in oocytes. This approach results in efficient transcript depletion and is highly adaptable...
Ujawnienia
The authors have nothing to disclose
Podziękowania
This research was supported in part by the University of Georgia, and a grant (HD071330) from the National Institutes of Health to MMV.
Materiały
| Name | Company | Catalog Number | Comments |
| Reagents | |||
| Pregnant Mare's Serum Gonadotropin (PMSG) | EMD Biosciences | 367222 | |
| Minimal Essential Medium (MEM) | *Recipe outlined in Table 1 | ||
| Earle's Balanced Salt Solution (10x) | Sigma | E-7510 | |
| Sodium Bicarbonate | Sigma | S-5761 | |
| Pyruvic Acid, sodium salt | Sigma | P-5280 | |
| Penicillin G, potassium salt | Sigma | P-7794 | |
| Streptomycin Sulfate | Sigma | S-9137 | |
| L-Glutamine | Sigma | G-8540 | |
| EDTA, disodium salt dihydrate | Sigma | E-4884 | |
| Essential Amino Acids (50x) | Gibco | 11130-051 | |
| MEM Vitamin Mixture (100x) | Sigma | M-6895 | |
| Phenol Red solution | Sigma | P-0290 | |
| Bovine Serum Albumin (BSA) | Sigma | A1470 | |
| Milrinone | Sigma | M4659 | |
| Fetal Bovine Serum (FBS) | Hyclone | SH30070.01 | |
| EmbryoMax M2 Media with Hepes | EMD Millipore | MR-015-D | |
| siRNAs targeting Pericentrin | Qiagen | GS18541 | |
| Negative control siRNAs | Qiagen | SI03650318 | |
| Paraformaldehyde (16% solution) | Electron Microscopy Sciences | 15710 | |
| Triton-X | Sigma | T-8787 | |
| Phosphate Buffered Saline (PBS) | Hyclone | SH30028.02 | |
| Anti-Pericentrin (rabbit) | Covance | PRB-432C | |
| Anti-acetylated a-tubulin (mouse) | Sigma | T-6793 | |
| Goat anti-rabbbit Alexa Fluor 488 | Invitrogen | A-21430 | |
| Goat anti -mouse Alexa Fluor 555 | Invitrogen | A-11017 | |
| Major Equipment | |||
| Stereomicroscope (SMZ 800) | Nikon | ||
| Upright Fluorescent Microscope | Leica Microsystems | ||
| Inverted Microscope | Nikon | ||
| Femtojet Micro-injections System | Eppenforf | ||
| Micro manipulators | Eppendorf | ||
| Micro-injection needles (femtotips) | Eppendorf | 930000035 | |
| Holding pipettes (VacuTip) | Eppendorf | 930001015 | |
| Plasticware | |||
| 35 mm culture dishes | Corning Life Sciences | 351008 | |
| 4-well plates | Thermo Scientific | 176740 | |
| 96 well plates | Corning Life Sciences | 3367 | |
| 0.45 mm CA Filter System | Corning Life Sciences | 430768 |
Odniesienia
- Nagaoka, S. I., Hassold, T. J., Hunt, P. A. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat Rev Genet. 13 (7), 493-504 (2012).
- Hassold, T. J., Hunt, P. A. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2 (4), 280-291 (2001).
- Szollosi, D., Calarco, P., Donahue, R. P. Absence of Centrioles in the First and Second Meiotic Spindles of Mouse Oocytes. J Cell Sci. 11 (2), 521-541 (1972).
- Manandhar, G., Schatten, H., Sutovsky, P. Centrosome Reduction During Gametogenesis and Its Significance. Biol Reprod. 72 (1), 2-13 (2005).
- Zimmerman, W. C., Sillibourne, J., Rosa, J., Doxsey, S. J. Mitosis-specific anchoring of gamma tubulin complexes by pericentrin controls spindle organization and mitotic entry. Mol Biol Cell. 15 (8), 3642-3647 (2004).
- Ma, W., Baumann, C., Viveiros, M. M. NEDD1 is crucial for meiotic spindle stabilty and accurate chromosome segregation in mammalian oocytes. Dev Biol. 339 (439-450), (2010).
- Ma, W., Viveiros, M. M. Depletion of pericentrin in mouse oocytes disrupts microtubule organizing center function and meiotic spindle organization. Mol Reprod Dev. 81 (11), 1019-1029 (2014).
- Bouniol-Baly, C., et al. Differential Transcriptional Activity Associated with Chromatin Configuration in Fully Grown Mouse Germinal Vesicle Oocytes. Biol Reprod. 60 (3), 580-587 (1999).
- De La Fuente, R., Eppig, J. J. Transcriptional Activity of the Mouse Oocyte Genome: Companion Granulosa Cells Modulate Transcription and Chromatin Remodeling. Dev Biol. 229 (1), 224-236 (2001).
- Hodgman, R., Tay, J., Mendez, R., Richter, J. D. CPEB phosphorylation and cytoplasmic polyadenylation are catalyzed by the kinase IAK1/Eg2 in maturing mouse oocytes. Development. 128 (14), 2815-2822 (2001).
- De La Fuente, R. Chromatin modifications in the germinal vesicle (GV) of mammalian oocytes. Dev Biol. 292 (1), 1-12 (2006).
- Wianny, F., Zernicka-Goetz, M. Specific interference with gene function by double-stranded RNA in early mouse development. Nat Cell Biol. , 270-275 (2000).
- Svoboda, P., Stein, P., Hayashi, H., Schultz, R. M. Selective reduction of dormant maternal mRNAs in mouse oocytes by RNA interference. Development. 127 (19), 4147-4156 (2000).
- Svoboda, P. Renaissance of mammalian endogenous RNAi. FEBS Letters. 588 (15), 2550-2556 (1016).
- Behringer, R., Gertsenstein, M., Nagy, K., Nagy, A. . Manipulating the Mouse Embryo: A Laboratory Manual. , (2014).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone