Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Repeatable Stair-step Assay to Access the Allelopathic Potential of Weedy Rice (Oryza sativa ssp.)
W tym Artykule
Podsumowanie
Allelopathy has shown promise as a useful supplemental weed control strategy in cropping systems. To determine the allelopathic potential of a desired plant specimen, a stair-step screening method is provided.
Streszczenie
Weed competition contributes significantly to yield losses in cropping systems worldwide. The evolution of resistance in many weed species to continuously applied herbicides has presented the need for additional management methods. Allelopathy is a physiological process that some plant species possess that provide the plant with an advantage over its neighbors. Allelopathic crop varieties would be equipped with the ability to suppress the growth of surrounding competitors, thus reducing potential yield loss due to weed interference. This paper focuses on the construction and operation of a stair-step assay used for the screening of the allelopathic potential of a donor species (Oryza sativa) against a receiver weed species (Echinochloa crus-galli) in a greenhouse setting. The structure described in this paper serves as a stand for the plant samples and incorporates a timed watering system for the accumulation and distribution of allelochemicals. Allelochemicals produced by the plant roots are allowed to flow downward through a series of four pots separately into a collection tank and recycled back to the top plant through electric pumps. This method of screening provides an avenue for the allelochemicals from the donor plant to reach receiver plants without any resource competition, thus allowing quantitative measurement of the allelopathic potential of the selected donor plant. The allelopathic potential is measurable through the height reduction of the receiver plants. Preliminary screening data for the effectiveness of this method demonstrated height reduction in the receiver species, barnyardgrass (E. crus-galli), and thus the presence of allelopathic residues from the donor plant, weedy rice (Oryza sativa).
Wprowadzenie
Allelopathy is a natural and complex phenomenon that has been the focus of many plant scientists in the past few decades. The mechanisms relating to allelopathy for use in crops have been the subject of much research since the 1930s, when Molisch observed that a plant has a direct or indirect effect on a neighboring plant through the production and secretion of chemical compounds into the environment1. Allelopathy is the production of secondary metabolites that have inhibitory effects on the growth and germination of some plant species. Released allopathic chemical compounds help provide the donor plants with a competitive advantage by adding phytotoxins to the environment around them2. Many factors contribute to the allelopathic activity. It is selective in its effectiveness and varies between varieties, environmental conditions, growth stage, stress, environment, and nutrient availability3.
In recent years, allelopathy has been highlighted in research as a possible supplement to the constant and growing weed control crisis. With the growing global population, the demand for sustainable food and fiber production has increased4. Weed control is one of the biggest threats to production faced by agronomists5,6. Traditional weed control methods focus on mechanical, chemical, and cultural practices. The continuous usage of herbicides, while effective, useful, and efficient, has promoted the evolution of resistant weed populations at an alarmingly fast pace7. Genetic engineering and breeding practices have been used effectively to give crops competitive advantages over weeds by designing them to withstand chemical applications that their neighbors cannot survive7,8. Although effective, these technologies are not always sustainable and sometimes pose outcrossing concerns9. Supplemental weed management practices need to be introduced if the goal of increasing food production is to be met10. Allelopathy shows excellent promise as a new defense tool for crops to improve their quality and outlive their competitors1,7.
Allelochemicals are often secondary products, and because their production is highly influenced by environmental factors, the specific compounds associated with plant suppression can be difficult to identify3. Production factors include genetics and the joint action of secondary metabolites that may act synergistically11,12. It is challenging to separate allelopathic activity from the competition that naturally exists within crop-weed interactions, and due to this, when screening for allelopathy there must be a standard set of outcomes that qualify the assay as valid and repeatable. Below is a set of criteria that qualifies findings of allelopathy as outlined by Olofsdotter et al.12 1) One plant must demonstrate suppression of another plant in a pattern; 2) The chemicals that are released into the environment in bioactive amounts must be produced by the donor plant; 3) The chemicals produced must be transportable to the receiver plant; 4) Some mechanism of uptake must be present in the receiver plant; 6) The pattern of inhibition observed must have no other exclusive explanation (e.g, competition for resources)12.
In an effort to overcome the barrier between the lack of knowledge of the mechanisms supporting allelopathy and variety development, phenotypic traits associated with allelopathic varieties can be identified and selected for further research and use. Some plants known to have allelopathic qualities are rye, sorghum, rice, sunflower, rapeseed, and wheat13. During the early observations of allelopathy in crops, due to distinguished borders of weed growth in field experiments, it was proposed that chemicals were involved rather than competition for resources14. However, most studies were field experiments that made it impossible to eliminate competition as a factor14. Competition elimination efforts gave way to lab and greenhouse experiments in attempts to prove and quantify allelopathic activity in rice and other crops. Field and greenhouse methods to screen plants for allelopathy demonstrate that allelopathic tendencies are present in both growing conditions11,15. Some critics believe that laboratory screenings may only hold limited value due to the lack of natural conditions, which may affect the results15.
The proposed method for screening allelopathic potential in plants provides adequate resources and space and eliminates resource competition with the use of a stair-step structure11,17. The method was adapted and modified from previous experiments exploring allelopathy in turfgrass and barley17,18. These studies found that a similar system was able to produce accurate results on the allelopathic potential of a target plant while removing any doubts that the observations could be attributed to natural competition. The stair-step method creates a circulatory system where a nutrient solution from a reservoir can cycle through each plant to an incubation tray through a few steps. An electric pump then recycles the solution along with any allelochemicals produced18. A method such as this is efficient in both time, space, and resources. It also provides similar field conditions for the plants and eliminates any resource competition. The methods and tools used for screening are easily manipulated to fit the desired study goals, conditions, and specific species. The objective of this study is to confirm weedy rice allelopathy through height suppression measurements on barnyardgrass with the use of the stair-step method.
Protokół
1. Stand Construction
NOTE: Measurements for the wood are listed as thickness (cm) x width (cm) x length (m).
- Cut wood into appropriate sizes and amounts as follows: five 10.16 cm x 5.08 cm x 0.91 m wooden pieces, three 10.16 cm x 5.08 cm x 0.76 m wooden pieces, three 10.16 cm x 5.08 cm x 0.61 m wooden pieces, five 10.16 cm x 5.08 cm x 0.46 m wooden pieces, three 10.16 cm x 5.08 cm x 0.3 m wooden pieces, and three 10.16 cm x 5.08 cm x 0.15 m wooden pieces.
- For the tallest level, stand one 2.44 m board across two 0.91 m pieces on each end at the edge and drill two screws vertically into each of the 0.91 pieces. Screw one more 0.91 m piece 1.22 m from each end for support, and place a 2.44 m board across the back of the 0.91 m stands and screw into place for support.
NOTE: The eight 3.175 cm x 15.24 cm x 2.44 m are kept as is and uncut to serve as the benchtop for each bench level. - Repeat step 1.2 for the next bench level with the 0.76 m pieces.
- Repeat step 1.2 for the next bench with the 0.61 m pieces down to the sixth bench at 0.15 m.
NOTE: No supporting 2.44 m board is needed for benches 3–6. The final stand has six benches with three vertical supports each, one on each end and one in the middle. - Line benches in descending height order with the overhanging lip facing the backside touching the bench above it, allowing for a gap between levels.
- Line a 0.91 cm board on each of the bottom edges of the benches along the ground and screw the benches in place.
- Screw a 0.46 m board horizontally for support on the tallest three benches on each side of the structure 0.61 m from the ground.
- Screw three corner braces onto the front-facing ends and center of the tallest bench.
- Screw one 2.54 cm x 5.08 cm x 20.32 cm wooden piece across the braces 2.54 cm from the base of the bench.
NOTE: Make one 0.91 m by 0.91 m by 2.44 m structure. Refer to Figure 1 for the final base product. Dimensions are subject to change with the experimental needs. The structure described was designed to fit 15.24 cm pots. The heights between benches were designed to fit the pots and plant material used in this experiment in order to maintain a steady flow of allelochemicals and solution from one pot to another down the benches by gravity.

Figure 1: Front view of the wooden base stand. A wooden base serves as the stand for the plant samples. Materials for the system are to be assembled and added depending on the number of samples needed for the experiment. In this study, two stands served as a base for 31 samples. Please click here to view a larger version of this figure.
2. System Assembly
- Remove the cap from a 1 L soda bottle and spray paint with black paint.
NOTE: The soda bottles will serve as a reservoir at the top of the system for one column. The paint provides a block for the light, decreasing or preventing algae growth. - At the bottom of each soda bottle, drill a small hole, just large enough to embed a 0.35 cm inner diameter (ID), 0.64 cm outer diameter (OD), 5.08 cm long plastic PVC tube.
- Smear a layer of silicone waterproof sealant around the edge of the hole after insertion to prevent any leaks. Let it dry completely.
- Repeat steps 2.2 and 2.3 on each of the plastic dishes used to hold the pots.
NOTE: Four dishes will be needed for one column. - Remove the lid and spray paint the outside of 2.27 L plastic canisters with black paint. These canisters will serve as the collection tanks at the base of each column.
- Drill a small hole in the upper backside of the canister.
NOTE: The supplies listed in steps 2.1–2.6 make one column. The number of columns is subject to the number of samples needed for the experiment desired. Two columns are needed for one sample. All dimensions are subject to change depending on the experimental needs. - After the supplies have been prepared and dried, place the soda bottle on the highest bench so that the PVC tube is hanging over the rim facing the stairs.
- Just below the soda bottle on the next bench, place one plastic dish with its tube hanging over the rim of the bench.
- Repeat step 2.8 for the next two benches.
- Place the canister on the bottom bench with the hole facing the back.
- Connect the canister with the dish above it by stringing the tube from the dish through the hole in the back of the canister.
- Smear waterproof sealant around the edge of the canister where the tube runs through to prevent leaks.
- Place a 21 W 1,000 L/hr submersible electric pump inside the bottom canister.
- Connect a 1.07 m long, 1.27 cm ID, 1.59 cm OD PVC tube to the nozzle of the electric pump.
- String the tube through the gap between the benches and over the back of the soda bottle at the top of the system.
- Plug the pump into a digital timer and set the timer setting as needed.
NOTE: The timer was set to run for 1 min every 3 h throughout the entire experiment. The selected timing allowed for the maximum amount of liquid in the collection tank to be cycled and allowed for approximately 10 min of flow each time the pump was turned on while avoiding flooding and spillovers.
3. Planting
- Sterilize all the rice seeds needed by rinsing in 70% ethanol for 30 s, soaking in 5% bleach for 20 min, and rinsing 6x with distilled water.
- Pregerminate the sterilized rice seeds in Petri dishes lined with filter paper filled with 5 mL of distilled water in a growth chamber set at 25 °C.
- After the seeds germinate, line the bottom of each pot with two large coffee filters by placing them inside the pots in their natural cupped form.
- Fill each pot to the top of the filter (approximately 75% of the pot) with autoclaved, washed, and screened specially graded quartz sand. Dampen the sand with distilled water by pouring water over the top of the sand or by placing pots in planting trays filled just slightly with distilled water to allow the pots to soak up the water and remain damp. Transplant six pregerminated donor plant seedlings into sand, evenly spaced.
- Cover the seedlings with sand.
- Let the seedlings establish for 3 weeks.
NOTE: The sand dries very quickly. Therefore, placing pots in trays is an efficient watering technique. Changing water out constantly will help prevent mold. - Pregerminate the receiver plant seedlings (E. crus-galli) in Petri dishes 3 weeks after planting donor plants by lining the bottom of the dish with filter paper and along with 5 mL of distilled water. Place the dishes in a growth chamber at 25 °C for 3–5 days.
- Prepare the pots as described in steps 3.1–3.2.
- After the seedlings germinate, transplant three seedlings into the prepared pots and cover with sand.
NOTE: The experiment begins one day after treatment (DAT), or the day that the receiver plant seedlings emerge and are transplanted and placed in the system.
4. Sample Placement
- Place four pots of one accession of donor plants in the four dishes of column 1, a single pot per row. Column 1 consists of donor plants only.
- Place two pots of the same accession of donor plants in the dishes of column 2 on the first and third row of the column.
- Place two pots of receiver plants in the dishes of column 2 on the second and fourth row in the column.
- For each replication, ensure that only one row of receiver plants is added. Two columns, the first consisting of donor plants only and the second alternating donors and receivers, make one treatment (Figure 2).
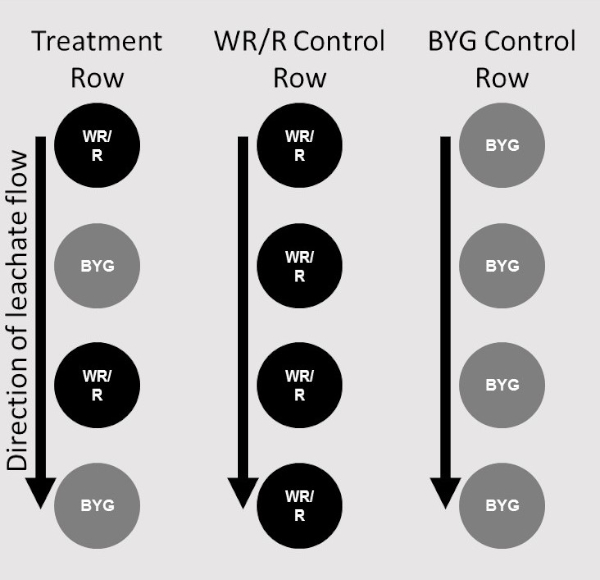
Figure 2: Placement map. Diagram depicting placements of donor (WR/R) and receiver plants (BYG) in respective positions in the stair-step system. Two columns of the stair-step system with plants in place comprise one treatment. A single column of receiver plants served as a control for one replication (far right), a single column of donor plants as a control for each accession (center), and the treatment column consisted of alternating donor and receiver plants (far left). Please click here to view a larger version of this figure.
- Repeat steps 4.1–4.4 for each treatment or donor plant accession (Figure 3).
NOTE: Each replication requires one column of receiver plant samples to serve as a control for one replication. Treatments were replicated 3x in a randomized complete block design.

Figure 3: Final stair-step structure. The stair-step system assembled with the plants in place. The system contained four rows of plant samples and a collection tank at the bottom for the solution to cycle to the top bottle and downward by gravity through each respective pot. Please click here to view a larger version of this figure.
5. Operation
- On DAT 1, fill the collection tank at the bottom of each column with half-strength Hoagland solution17 in distilled water, approximately 1,500 mL.
- Set the timers to run as desired in the auto off setting.
- Cover the collection tanks with black plastic to limit light exposure and evaporation.
- Fill the tanks every 2 days with 500 mL of Hoagland's solution to keep the system flowing constantly.
- Maintain the greenhouse temperatures at 28 °C during the day and 24 °C at night respectively with a 16/8 h split and humidity at 53%.
6. Data Collection
- Measure and record the heights of each plant in the stair-step system on DAT 1 and once every week up to DAT 21 by placing a ruler at the base of each plant and observing the tallest leaf stand.
- Measure and record the chlorophyll levels of each plant on DAT 7 and 14 using the chlorophyll content meter.
- On the last day of the experiment (i.e., DAT 21) label one paper bag for each pot.
- Cut plant samples at the base and place in separate bags.
- Place all samples in an oven dryer set at 60 °C for 48 h16.
- Remove dried samples and empty contents individually onto a scale and record the weight in grams.
7. Data Analysis
- Calculate the allelopathic potential of the donor plants based on the percent inhibition of the receiver plant using this equation:
height reduction (%) = [height of control (cm) – height of treated (cm)] × 100 - Calculate the donor plant height reduction as a check for any reverse effect the receiver plant may have on the target plants.
- Analyze accessions as the fixed effect while replications and runs are the random effects18.
- Analyze the data using a general linear model with mean values separated using Fisher's protected least significant difference at or below a 0.05 probability level in a statistical software (e.g., JMP 14).
- Visualize the correlation among the original variables using principle component analysis of by uploading data.
- Select the Analyze tab in the toolbar, select Fit Y by X. Under columns, Highlight the response (i.e., percent height reduction) then click Y, response to specifythe factor being observed for Y, (i.e., percent height reduction). For the X factor, Hightlight accession and click X, factor, then select OK.
- Select the red down arrow on the Oneway Analysis bar, select Means/ANOVA. Again select the down arrow on the Oneway Analysis bar and highlight compare means then select each pair, student's T.
Wyniki
Two preliminary screenings using this method were performed on nine weedy rice accessions (B2, S33, B83, S97, S94, B81, B8, B34, B14) and five cultivated rice lines (PI338046, Rex, Rondo, PI312777, CL163). Weedy rice accessions and rice lines were selected based on their performance in previous allelopathic screenings conducted by Shrestha (2018)18. The weedy rice seeds were collected from across the state of Arkansas. The rice lines selected are commonly grown lines in the US, some known to expre...
Dyskusje
Exploiting allelopathy may potentially serve as a biological control for weeds that are difficult to manage1,7,13. Allelopathy has shown great potential as a possible solution to the weed crisis in rice and serves as an alternative or supplement to chemicals and manual weed control practices5,13,19. Identifying allelopathic varieties or...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
Funding for this project was provided by the Special Research Initiative Grant sponsored by the Mississippi Agricultural and Forestry Experiment Station and is based upon work that is supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, Hatch project under accession number 230060.
Materiały
| Name | Company | Catalog Number | Comments |
| 1.25 in by 6 in by 8 ft standard severe weather wood board | Lowe's, Mooresville, NC | 489248 | N/A |
| 2 in by 4 in by 8 ft white wood stud | Lowe's, Mooresville, NC | 6005 | Cut into appropriate sizes |
| 63 mm (2.5 in) corner braces | Lowe's, Mooresville, NC | 809449 | N/A |
| Asporto 16 oz Round Black Plastic To Go Box - with Clear Lid, Microwavable – 6.25 in by 6.25 in by 1.75 in - 100 count box | Restaurantware.com, Chicago, IL | RWP0191B | black |
| ATP vinyl-flex PVC food grade plastic tubing, clear, 0.125 in id by 0.25 in od, 100 ft | Amazon, Seattle WA | B00E6BCV0G | N/A |
| Ccm-300 chlorophyll content meter | Opti-Sciences, Inc. Hudson, NH | ccm/300 | N/A |
| Common 1 in by 2 in by 8 ft pine board | Lowe's, Mooresville, NC | 1408 | N/A |
| Contractors choice contractor 24-pack 42-gallon black outdoor plastic construction trash bag | Lowe's, Mooresville, NC | 224272 | Cut to cover collection tanks |
| EURO POTS | Greenhouse Megastore, Danville, IL | CN-EU | 15 cm short black 6 in diameter 4.25 in height 1.37 qt volume |
| Fisher brand petri dish with clear lid | Fisher Scientific, Waltham, MA | FB0857513 | N/A |
| Aexit Ac 220 V-240 V electrical equipment US plug 21 W 1,000 L/hr multipurpose submersible pump | Amazon, Seattle WA | B07MBMYQNT | Nozzle size should fit tubes and can be repaced |
| Woods 50015 WD outdoor 7 day heavy-duty digital outlet timer | Walmart, Bentonville, AR | 565179767 | 20 settings |
| GE silicone 2+ 10.1 oz almond silicone caulk | Lowe's, Mooresville, NC | 48394 | Sealant for edges of any attached tubing |
| Great Value Distilled Water | Walmart, Bentonville, AR | 565209428 | N/A |
| Great Value White Basket coffee filters 200 count | Walmart, Bentonville, AR | 562723371 | Size may vary |
| Grip-rite primgaurd plus #9-3 in pollimerdex screws | Lowe's, Mooresville, NC | 323974 | N/A |
| Hoagland’s No. 2 basal salt mixture | Caisson Laboratories, INC. Smithfield, UT | HOP01/50LT | ½ strength rate |
| JMP (14) | SAS Institute Inc. North Carolina State University, NC | N/A | |
| Project source flat black spray paint | Lowe's, Mooresville, NC | 282254 | N/A |
| Project source utility 1.88 in by 165 ft gray duct tape | Lowe's, Mooresville, NC | 488070 | N/A |
| Rubbermaid 2 qt square food storage canister clear | Walmart, Bentonville, AR | 555115144 | Collection tank discard lid |
| Sealproof unreinforced PVC clear vinyl tubing, food-grade .5 in id by .625 in od, 100 ft | Amazon, Seattle WA | B07D9CLGV3 | Connects to pump |
| Short Mountain Silica 50 lb Play sand | Lowe's, Mooresville, NC | 10392 | Sand should be purified |
| Steve Spangler's 1 L Soda Bottles - 6 Pack - For Science Experiment Use | Amazon, Seattle WA | UPC 192407667341 | Top step tank discard lid |
Odniesienia
- Weston, L. A. History and Current Trends in the Use of Allelopathy for Weed Management. HortTechnology. 15 (3), 529-534 (2005).
- Pratley, J. E. Allelopathy in annual grasses. Plant Protection Quarterly. 11, 213-214 (1996).
- Bertin, C., Yang, X., Weston, L. A. The role of root exudates and allelochemicals in the rhizosphere. Plant and Soil. 256 (1), 67-83 (2003).
- Stevenson, G. R. Pesticide Use and World Food Production: Risks and Benefits. Environmental Fate and Effects of Pesticides. American Chemical Society. , 261-270 (2003).
- Chopra, N., Tewari, G., Tewari, L. M., Upreti, B., Pandey, N. Allelopathic Effect of Echinochloa colona L. and Cyperus iria L. Weed Extracts on the Seed Germination and Seedling Growth of Rice and Soybean. Advances in Agriculture. 2017, 1-5 (2017).
- Jabran, K., Mahajan, G., Sardana, V., Chauhan, B. S. Allelopathy for weed control in agricultural systems. Crop Protection. 72, 57-65 (2015).
- Worthington, M., Reberg-Horton, C. Breeding Cereal Crops for Enhanced Weed Suppression: Optimizing Allelopathy and Competitive Ability. Journal of Chemical Ecology. 39, 213-231 (2013).
- Sudianto, E., et al. Corrigendum to "Clearfield (R) rice: Its development, success, and key challenges on a global perspective.". Crop Protection. 55, 142-144 (2014).
- Gressel, J., Valverde, B. E. A strategy to provide long-term control of weedy rice while mitigating herbicide resistance transgene flow, and its potential use for other crops with related weeds. Pest Management Science. 65, 723-731 (2009).
- Muthayya, S., Sugimoto, J. D., Montgomery, S., Maberly, G. F. An overview of global rice production, supply, trade, and consumption. Annals of the New York Academy of Sciences. 1324, 7-14 (2014).
- Chung, I. M., Kim, K. H., Ahn, J. K., Lee, S. B., Kim, S. H. Allelopathy Comparison of Allelopathic Potential of Rice Leaves, Straw, and Hull Extracts on Barnyardgrass. Agronomy Journal. 95 (4), 1063-1070 (2003).
- Olofsdotter, M., Jensen, L. B., Courtois, B. Improving crop competitive ability using allelopathy Ð an example from rice. Journal of Plant Breeding. 121, 1-9 (2002).
- Olofsdotter, M., Navarez, D., Rebulanan, M., Streibig, J. C. Weed-suppressing rice cultivars-does allelopathy play a role. Weed Research. 39 (6), 441-454 (1999).
- Jensen, L. B., et al. Locating Genes Controlling Allelopathic Effects against Barnyardgrass in Upland Rice. Agronomy Journal. 93 (1), 21-26 (2001).
- Kuijken, R. C., Eeuwijk, F. A. V., Marcelis, L. F., Bouwmeester, H. J. Root phenotyping: from component trait in the lab to breeding. Journal of Experimental Botany. 66 (18), 5389 (2015).
- Lickfeldt, D. W., Voigt, T. B., Branham, B. E., Fermanian, T. W. Evaluation of allelopathy in cool season turfgrass species. International Turfgrass Society. 9, 1013-1018 (2001).
- Liu, D. L., Lovett, J. V. Biologically active secondary metabolites of barley: Developing techniques and assessing allelopathy in barley. Journal of Chemical Ecology. 19, 2217-2230 (1993).
- Shrestha, S. . Evaluation of Herbicide Tolerance and Interference Potential among Weedy rice germplasm. , (2018).
- Kim, K. U., Shin, D. H., Olofsdotter, Rice allelopathy research in Korea. Allelopathy in Rice. IRRI. , (1998).
- Quasem, J. R., Hill, T. A. On difficulties with allelopathy. Weed Research. 29, 345-347 (1989).
- Singh, S., et al. Evaluation of mulching, intercropping with Sesbania and herbicide use for weed management in dry-seeded rice (Oryza sativa L.). Crop Protection. 26, 518-524 (2007).
- Kong, C. H., Li, H. B., Hu, F., Xu, X. H., Wang, P. Allelochemicals released by rice roots and residues in soil. Plant and Soil. 288 (1-2), 47-56 (2006).
- Ervin, G. N., Wetzel, R. G. Allelochemical autotoxicity in the emergent wetland macrophyte Juncus effusus (Juncaceae). American Journal of Botany. 87 (6), 853-860 (2000).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone