Method Article
A Modified Method for Intrathecal Catheterization in Rats
W tym Artykule
Podsumowanie
Herein, we introduce a modified method for intrathecal catheterization in rats that represents a simple, convenient, and reliable approach for repetitive intrathecal drug administration.
Streszczenie
Intrathecal catheterization has been widely applied in animal experiments, especially those on neuropathic pain. However, the traditional methods still have several limitations. Although some investigators have attempted to improve the traditional methods, the available methods still need to be modified. Herein, we introduce a modified method for intrathecal catheterization in rats.
This method uses a 20 cm long stainless-steel wire (0.2 mm in diameter), a 15 cm long plastic PE10 tube, a self-made sealing cap, and a 0.3 cm × 0.5 cm anti-allergic band. Our modified method for intrathecal catheterization has several advantages. First, introducing a stainless-steel wire to PE10 tube increases the elasticity of the tube, improves the success rate of intrathecal catheterization, reduces the amount of space required for the operation, and minimizes the damage to the tissues around the lumbar spine. Second, the length of PE10 tube is determined before the surgery, and catheter indwelling time can be longer than one week. Third, the PE10 tube is fixed by a figure-8 suture, 4 times, which prevents tube movement and retraction when the animal moves. Fourth, a self-made sealing cap is used to seal the PE10 tube, which not only prevents cerebrospinal fluid leakage but also reduces the need for repeated cutting of PE10 tube. Finally, the extracorporeal end of PE10 tube is tied with a band, which prevents tube retraction when the animal moves.
This method can increase the catheterization success rate in rats, as approximately 80% of PE10 tubes remained in place even 28 days after surgery. Thus, this modified method may represent a simple, convenient, and reliable approach for repetitive intrathecal drug administration.
Wprowadzenie
Intrathecal catheterization (also known as subarachnoid catheterization) in rats is a method that involves inserting a catheter into the subarachnoid space through the intervertebral space1. Drugs are directly injected into the subarachnoid space through the catheter, which helps researchers investigate the effects of drugs on the spinal cord without considering the effects of drugs that penetrate the blood-brain barrier2,3. Moreover, cerebrospinal fluid can be collected following intrathecal catheterization to investigate the microenvironment of the central nervous system4,5. The currently used method for intrathecal catheterization was first established by Yaksh and Rudy6 in 1976, and since then, it has been widely applied in animal experiments in the fields of neuroscience, anesthesia and analgesia, spinal cord-mediated cardiovascular regulation, and especially neuropathic pain2,7. However, this method still has several limitations, such as a high incidence of spinal cord damage, subarachnoid hemorrhage, postoperative sensory and motor dysfunction, high postoperative mortality, and a high risk of neurological impairment4,5,8,9,10. In an attempt to overcome these limitations, catheterization of the subarachnoid space through lumbar interspaces was proposed by Størkson et al. in 199611, and a higher postsurgical success rate was reported. Notably, the fixation of indwelling catheter is still a challenge in this method, and catheter retraction is common due to animal movement, which makes intrathecal drug administration inconvenient.
Because of the above limitations, some investigators12,13,14,15 have attempted to improve the tools for puncturing, the methods of catheterization, and the methods of catheter fixation, but the available methods still need to be modified due to the difficulty in quantifying the diameter of the beads used, the need for repeated punctures and the short length of the catheter, etc.11
According to the lumbar approach for intrathecal catheterization1 and the Seldinger technique for central vein catheterization,16 we developed a method for intrathecal catheterization in rats that uses a stainless-steel wire, a self-made sealing cap, and an antiallergic band to simplify the existing method. Through this method, the catheter can be easily inserted into the subarachnoid space and stably fixed on the back of the rat, and the need for repeated punctures for repeated intrathecal drug administration is avoided.
Herein, we introduce a modified method that may improve the success rate of intrathecal catheterization in rats and represent a simple, convenient, and reliable approach for repetitive intrathecal administration of drugs.
Protokół
Intrathecal catheterization was carried out in strict accordance with the recommendations in the Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health, and the protocol was approved by the Ethics Committee of Experimental Animals, China (No. TJBH15523201). Male Sprague-Dawley (SD) rats were used in the experiment. Care was taken to minimize the pain and discomfort of the animals.
1. Material and instrument preparation
NOTE: The preparation of materials and instruments is very important for successful intrathecal catheterization.
- Prepare a 15 cm long PE10 tube (the length is determined according to the distance between the rat head and the end of the tail), insert a 20 cm long stainless-steel wire (0.2 mm in diameter) with two polished ends into the PE10 tube as a support, and mark the tube 2 cm from one end to indicate the insertion depth (as marked by black crosses in Figure 1A,B).
- Cut the sharp tip of a 22 G needle and seal the distal end (Figure 1C).
- Cut an epidural catheter (1.0 mm in external diameter) into 1 cm fragments. Then, insert a fragment into a sharp-tip-free 22 G needle (Figure 1D), and seal the distal end of the fragment with a pair of heated straight forceps. This apparatus is called the tube sealing cap (Figure 1E) .
- Prepare a 0.3 cm × 0.5 cm antiallergic band by cutting a silk tape (1.25 cm × 9.1 m) with a pair of scissors (Figure 1F).
2. Preparation for surgery
- Prepare the instruments for intrathecal catheterization by sterilizing them before surgery. The instruments used for the surgery are toothed forceps, scissors, a gavage apparatus, a scalpel handle and #10 blades. (Figure 2).
- Immerse the PE10 tube and guide wire in 75% ethanol for sterilization for approximately 2 h.
3. Surgery
- Anesthetize the rat with 3% isoflurane at a flow rate of 3 L/min.
- Place the rat on the operating table and observe the withdrawal reflex when pinching the hind paw with forceps. The absence of hind paw movement in response to stimulation confirmed successful anesthesia. Administer adequate analgesia by intramuscular injection of 1 mg/kg meloxicam before intrathecal catheterization.
- Remove hair from the lumbar spine region of the back and the area between two ears with a shaver.
- Place a centrifuge tube (3 cm in diameter) under the abdomen of the rat at the waist-hip junction to increase the flexion in the lumbar spine, giving more room for the needle and the catheter to pass through.
- Sterilize the surgical sites (the area over the lumbar spine region and the area between two ears) with povidone-iodine solution and then with ethanol solution three times. Cover the rat with an aseptic dressing, and expose the surgical sites. Then wash the PE10 tube and guide wire with normal saline before surgery.
NOTE: The tail was not covered so that tail movement could be observed during intrathecal catheterization. - Determine the location of the intervertebral space between L5 and L6 by locating the spinous process of L6 at the midpoint between the left and right bilateral iliac crests. Fix the skin with the left thumb and the left index finger of the operator, and then make a 3-4 cm long midline incision just above the spinous process between L4 and S1.
- Bluntly separate the subcutaneous tissues with a pair of scissors. Locate the intervertebral space between L5 and L6 again and make a small incision (0.3 - 0.5 cm) on both sides of the L5 and L6 dorsal processes.
- Clamp and lift the L5 dorsal process with a pair of toothed forceps to expand the intervertebral space. Then, bluntly separate the muscles around the vertebral body with a pair of scissors until the top of the L6 dorsal process is completely exposed.
NOTE: Removal of any part of the vertebral body and muscles should be avoided, with the aim of minimizing damage to surrounding tissues. - When the L5 dorsal process is lifted with a pair of toothed forceps and the intervertebral space is expanded with another pair of forceps, clean the L5-6 intervertebral space with a cotton ball until the inverted "V" area is completely exposed.
- Puncture the spine with a 23 G needle in the inverted "V" area just under the top of the L6 dorsal process.
NOTE: A tail flick is observed, and/or colorless transparent fluid flows out from the subarachnoid space, indicating a successful puncture into the subarachnoid space. - Carefully insert the PE10 tube containing stainless-steel wire into the spinal canal at the puncture site, being tilted 30° toward the tail. Adjust the insertion angle until the PE10 tube can be successfully inserted without resistance (a tail flick was observed during this process).
- When the marked area of the PE10 tube reaches the posterior muscle, catheterization is stopped.
- Slowly remove the stainless wire from the PE10 tube. A tail flick may be observed.
NOTE: A tail flick may be observed, and after the wire is removed, transparent fluid (or light red fluid) may flow out of the tube. - Then, connect the PE10 tube to a 1 mL syringe, through which 20 µL of normal saline is injected. After the syringe is removed, saline will flow continuously out of the PE10 tube, indicating that it has been successfully inserted into the subarachnoid space.
- Once the PE10 tube is confirmed to be unobstructed, suture the muscles on one side of the vertebral body with a 4-0 suture and make a knot. Then, tie the suture around the PE10 tube and make another knot. Do not cut the suture; suture the muscles on the other side; tie the suture to the PE10 tube again, make a third knot, and cut the suture.
NOTE: This process fixes PE10 tube with a figure-8 suture to reduce the possibility of displacement and retraction of the tube. - Make a 0.5 cm long incision 1 cm below the midpoint between the ears. Bluntly separate the subcutaneous tissues with scissors, and insert a metal gavage tube toward the tail until the tip is visible in the lumbar incision.
- Insert the distal end of the PE10 tube into the gavage tube until the PE10 tube exits the other end of the gavage tube; then gently withdraw the gavage.
- When the PE10 tube is confirmed to be unobstructed again, suture the remaining muscles around the lumbar incision with a 4-0 suture, tie the suture around the PE10 tube, and make another knot to fix the PE10 tube again.
- Suture the skin, avoiding damage to the PE10 tube. Then, suture the neck skin with a 4-0 suture, tie the suture around the PE10 tube, and make a knot to fix the PE10 tube.
- When the PE10 tube is confirmed to be unobstructed again, seal the extracorporeal end of the PE10 tube with a sealing cap.
- Dry the PE10 tube with a piece of tissue and then tie the antiallergic band around the PE10 tube several times to prevent retraction of the PE10 tube during rat movement.
4. Lidocaine validation experiment
- After the surgery, return the rat to its cage (one per cage) and closely monitor it during the recovery from anesthesia until the rat gains consciousness.
- After the rat is fully awake, remove the sealing cap and inject 20 µL of 2% lidocaine into PE10 tube at a rate of 0.02 mL/s via a Hamilton syringe, followed by the injection of 10 µL of normal saline.
- Seal the PE10 tube with the sealing cap.
- Place the rat on a table and observe carefully. The presence of hind limb paralysis after an intrathecal injection of lidocaine (from PE10 tube) indicates successful catheterization (Figure 3). Hind limb paralysis usually lasts approximately 30 min10.
NOTE: Allow the rat to recover for 5-7 days before the following experiments. - Closely monitor the rat during the recovery period until complete recovery of limb function.
Wyniki
For intrathecal injection, the extracorporeal tip of PE10 tube was cut off, and PE10 tube was sealed with a sealing cap between two drug injections. In our pilot study, the success rate of intrathecal catheterization was approximately 95% (19 out of 20 rats); success was indicated by a tail flick and/or the release of colorless transparent fluid during the procedure. Approximately 85% of the tubes remained in place 7 days after surgery, and approximately 80% remained in place 28 days after surgery. The rats recovered soon after the operation, and no complications were observed within 7 days after surgery. The daily movement was normal, and behavioral abnormality was not observed. These results indicate our method is superior to those previously reported in terms of the success rate and long-indwelling rate.
Complete paralysis of the lower limbs upon lidocaine injection via catheters indicates successful intrathecal catheterization15. The success rate of intrathecal catheterization is calculated by dividing the total number of rats by the number of rats with successful catheterization. With our modified method, the success rate was 95%, which was greater than the rate achieved with the method reported by Hou et al. (88%)15. This is shown in Figure 4.
The intrathecal tube was monitored at 2, 5, and 7 days after intrathecal catheterization and the rate of successful indwelling catheter was calculated as number of rats with successful indwelling catheter/total number of rats × 100%. At 2, 5, and 7 days after intrathecal catheterization, the rate of successful indwelling catheter was 94%, 81%, and 65%, respectively, in the study of StØrkson et al.11. The rate of successful indwelling catheter at 2, 5 and 7 days after intrathecal catheterization was 95%, 90% and 85%, respectively, with our technique (Figure 5).

Figure 1. Materials and instruments used for intrathecal catheterization. (A) A 15-cm long PE10 tube was prepared, and the tube was marked 2 cm from one end to indicate the insertion depth. (B) A 20-cm long stainless-steel wire with two polished ends was inserted into the PE10 tube as a support. (C) The sharp tip of the 22G needle was cut with a pair of scissors, and the distal end was sealed with a pair of forceps. (D) An epidural catheter (1.0 mm in external diameter) was cut into 1-cm fragments, which were then inserted into the sharp-tip-free 22G needle. (E) The distal end of the epidural catheter was sealed with a pair of heated straight forceps; this apparatus was called the tube sealing cap. (F) A 0.3 cm × 0.5 cm anti-allergic band (silk tape, 1.25 cm × 9.1 m) was prepared with a pair of scissors. Please click here to view a larger version of this figure.
Figure 2. Preparation of instruments for intrathecal catheterization. Instruments (such as toothed forceps, scissors, a gavage apparatus, a scalpel handle, and #10 blades) were sterilized with ethanol for approximately 2 h and then washed with normal saline approximately 30 min before surgery. Please click here to view a larger version of this figure.
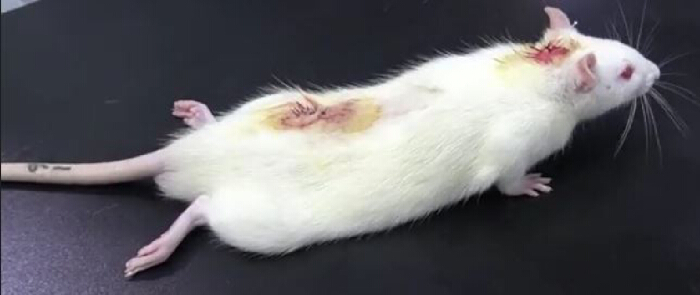
Figure 3. Results of the lidocaine validation experiment after intrathecal catheterization. After intrathecal injection of 20 µL of 2% lidocaine followed by injection of 10 µL of normal saline, the rat was temporarily paralyzed: lower limb paralysis occurred within 30 s and disappeared 30 min later, indicating successful intrathecal catheterization. Please click here to view a larger version of this figure.
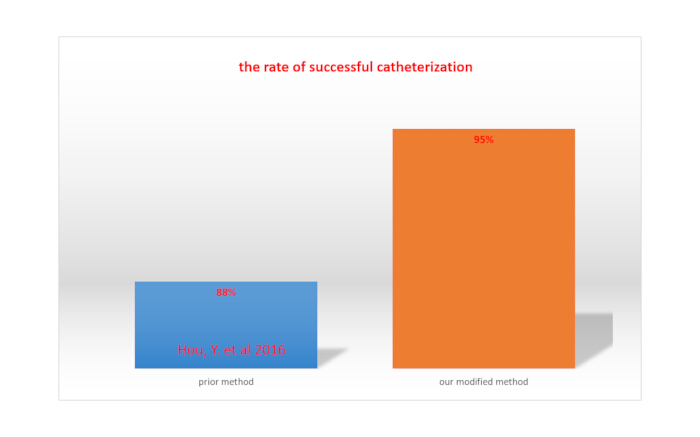
Figure 4. Comparison of the rate of successful catheterization between our modified method and a previously reported method. Please click here to view a larger version of this figure.
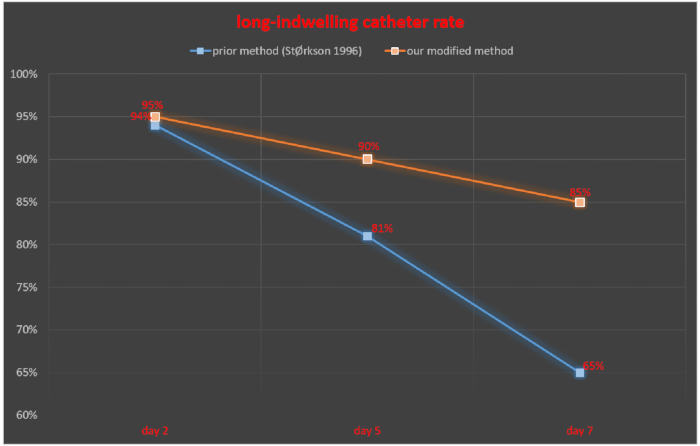
Figure 5. Comparison of the long-indwelling catheter rate between our modified method and a previously reported method. Please click here to view a larger version of this figure.
| Modified method | Prior method | Advantages of modified method | Disadvantages of prior method | |
| Guiding method for insertion | a stainless-steel wire | Guide-canula (20G 0.9× 38 mm) | Increases the elasticity of the tube, | The resistance is difficult to feel, increasing the difficulty of operation |
| Improves the success rate of intrathecal catheterization | Damage to the tissues due to repeated puncture | |||
| Reduces the requirement of operative space | One end of the tube is stretched to 1.5 times original length, making the diameter of both ends different | |||
| Minimizes the damage to tissues around the lumbar spine | Susceptibility to cerebrospinal fluid leakage because the diameter of 20G guide-canula is 2 times or more that of stretched PE10 tube | |||
| Length of PE10 tube | 15 cm | 14 or 28 cm | Easy to determine the length of PE10 tube regardless of the duration of catheter indwelling | Duration of catheter indwelling is shorter for shorter PE10 tube; susceptibility to falling out of the body for long PE10 tube |
| Fixation method | "8" suture and 4 times | 1 or 2 beads | Avoids the tube movement and retraction during the animal activities | Difference in the diameter of the tube at both ends, and susceptibility to displacement of PE10 tube during the bead-making |
| Method for tube sealing | Self-made cap | No | Prevents the leakage of cerebrospinal fluid | Requirement of repeated cutting of PE10 tube |
| Avoids the repeated cutting of PE10 tube | ||||
| Method for prevention of retraction | Anti-allergic band | 1 or 2 beads | Prevents the tube from retraction during the animal activities | Susceptibility to retraction |
Table 1. Advantages and disadvantages of the modified method and a previously reported method.
Dyskusje
There are several critical tips for this modified method to maximize the success rate of intrathecal catheterization. First, a 20 cm long stainless-steel wire with two polished ends should be prepared and inserted into PE10 tube as a support. Second, the operator should completely expose the inverted "V" area after cleaning the L5-6 intervertebral space with a cotton ball, and the intervertebral space should be expanded with another forceps while lifting the L5 dorsal process with toothed forceps. Third, PE10 tube should be fixed with a figure-8 suture four times. Finally, the extracorporeal end of PE10 tube should be tied with a band and sealed with a self-made cap.
The success rate of intrathecal catheterization and damage to tissues around vertebral bodies may significantly influence the reliability of the experimental results15. Thus, improving the success rate as much as possible and reducing the damage to the surrounding tissues are crucial in establishing animal models and relevant experiments1. In this modified method, a stainless-steel wire is inserted into PE10 tube for guidance, which increases the elasticity of the tube and improves the success rate of intrathecal catheterization. Moreover, the amount of space needed for operation is reduced with this modified method, and the damage to the tissues around the lumbar spine is minimized because the surrounding tissues are bluntly separated, but not cut. In comparison, in the previously reported method11, a 20 G guide cannula is used to reduce resistance during puncturing, and repeated puncturing is often needed, which may injure the tissues. In addition, in the previously reported method, to reduce the diameter of PE10 tube, it is immersed in warm water (60 °C) and then stretched at one end to approximately 150% of the original length, which may not ensure the consistency of tube diameter and therefore may cause leakage of cerebrospinal fluid because the diameter of 20G guide cannula is approximately two times greater than or equal to that of the stretched PE10 tube. Moreover, in our method, lumbar function is preserved to the greatest extent, which avoids the influence of surgery on the results of subsequent experiments. These results are consistent with those reported by Xu et al2.
In previously reported method11, the length of PE10 tube is approximately 14 cm if the tube is fixed at the site around the puncture site, but the catheter indwelling time is often shorter than 7 days under these conditions (or the tube is removed from the body by the rat). The length of PE10 tube is approximately 28 cm if the tube is fixed at the back of the neck, which is significantly longer than the PE10 tube used in our method (15 cm). Although beads were formed following the protocol reported by Størkson et al.11, some tubes were removed from the body, and only approximately 65% of the tubes were still fixed in place at 7 days after surgery, which significantly affected the outcomes of subsequent experiments. In our method, PE10 tube is fixed with a figure-8 suture 4 times, and the extracorporeal end of PE10 tube is tied with a band to reduce the possibility of displacement and retraction. Following our method, approximately 85% of the tubes remained in place at 7 days after surgery, and approximately 80% of the tubes remained in place at 28 days after surgery.
In the previously reported method11, the extracorporeal tip of the intrathecal catheter should be cut off for each drug administration. However, the repeated intrathecal administration of drugs may shorten the catheter indwelling time, which makes the intrathecal administration of drugs inconvenient. Therefore, in our method, a self-made cap is used to seal PE10 tube, which is sterilized with ethanol once daily. This not only prevents the leakage of cerebrospinal fluid but also reduces the need for repeated cutting of PE10 tube for intrathecal administration of drugs, ensuring the effective delivery of the drugs.
The advantages and disadvantages of the modified method and previously reported method are summarized in Table 1. First, for the modified method, the use of stainless-steel wire in PE10 tube increases the elasticity of the tube and improves the success rate of intrathecal catheterization, the amount of space needed for the operation is reduced, and the damage to the tissues around the lumbar spine is minimized. In previously reported method, a 20G guide cannula is inserted until resistance is felt, and repeated puncture is often needed, which may result in damage to tissues. In addition, the PE10 tube at one end is stretched until its length reaches approximately 150% of original length, which may cause cerebrospinal fluid leakage because the diameter of the 20G guide cannula is 2 times greater than or equal to that of stretched PE10 tube. Second, in the modified method, the length of PE10 tube is determined before surgery, and the catheter indwelling time can be longer than one week. In previously reported method, the length of PE10 tube is approximately 14 cm if it is fixed at the puncture site, but the catheter indwelling time is often shorter than 7 days because the tube is susceptible to being pulled out of the body by the rat; the length of PE10 tube is approximately 28 cm if it is fixed at the back of the neck, which is significantly longer than the length of the tube used in our method. Third, in the modified method, the PE10 tube is fixed with a figure-8 suture 4 times to prevent tube movement and retraction; a self-made cap is used to seal PE10 tube, which not only prevents leakage of cerebrospinal fluid but also prevents the need for repeated cutting of PE10 tube. In the previously reported method, it is difficult to obtain beads with a consistent diameter, the displacement of PE10 tube is common when beads are formed, and repeated cutting of PE10 tube is often needed. Finally, in the modified method, the extracorporeal end of PE10 tube is tied with a band, which prevents the tube from retracting during movement. However, in the prior method, the beads cannot reliably prevent PE10 tube retraction because it is difficult to obtain beads with a consistent diameter.
Overall, this modified method for intrathecal catheterization has the following advantages. First, the use of stainless-steel wire in PE10 tube increases the elasticity of the tube and improves the success rate of intrathecal catheterization, the amount space needed for the operation is reduced, and damage to the tissues around the lumbar spine is minimized, which preserves the lumbar function to the greatest extent possible and avoids the influence of surgery on the results of subsequent experiments. Second, PE10 tube is fixed with a figure-8 suture 4 times, which prevents tube movement and retraction during movement. Third, a self-made sealing cap is used to seal PE10 tube, which not only prevents cerebrospinal fluid leakage but also prevents the need for repeated cutting of PE10 tube. Repeated cutting of the catheter may shorten the catheter, which makes the delivery of drugs inconvenient. Finally, the extracorporeal end of PE10 tube is tied with an antiallergic band, which prevents the tube from retracting during movement.
However, there are several limitations in this modified intrathecal catheterization technique. First, after surgery, the rats need to be housed separately (one per cage) to avoid damage to the extracorporeal end of PE10 tube. Second, recovery for 5-7 days after intrathecal injection of lidocaine is needed before subsequent experiments.
In conclusion, this modified method for intrathecal catheterization may serve as a useful tool for the repetitive intrathecal administration of drugs and represent a simple, convenient, and reliable way to shorten the duration of experiments.
Ujawnienia
The authors of this manuscript declare that there are no conflicts of interest.
Podziękowania
This work was supported by the National Natural Science Foundation (No. 81971042) and the Key Support Specialist Projects of Shanghai Hongkou District Health Commission (No. HKZK2020A06).
Materiały
| Name | Company | Catalog Number | Comments |
| 1 cc syringe | Jiangxi Hongda Medical Equipment Co., Ltd | 1 cc | |
| 22 gauge × 1” needles | Jiangxi Hongda Medical Equipment Co., Ltd | 22G | |
| 23 gauge × 1” needles | Jiangxi Hongda Medical Equipment Co., Ltd | 23G | |
| 25 μL Hamilton Syringes | Shanghai Bolige Co.,Ltd | 0.31mm 25 μL | |
| 4-O MERSILK NON-ABSORBABLE SUTURE | ETHICON | SA83G | |
| 50 mL corning centrifuge tubes 3 cm diameter | 430820 | CORNING | |
| Epidural catheter and connector | Henan Tuoren Medical Device Co., Ltd | regular type | |
| Gavage apparatus | Shanghai Bolige Co.,Ltd | 8# | |
| PE-10 Mirco Medical Tubing | BB31695-PE/1 | Scientific Commodities, Inc | |
| Scalpel handle and #10 blades | Jiangsu Songxin Medical Equipment Co., Ltd | 125mm | |
| Scissors | Jiangsu Songxin Medical Equipment Co., Ltd | 100mm | |
| Sprague-Dawley (SD) rats | Shanghai BK/KY Biotechnology Co., Ltd | Male | |
| Stainless steel wire 0.2 mm diameter | Dongguan Jiazhi Metal Products Technology Co., Ltd. | 0.2mm × 1m | |
| Toothed forceps | Jiangsu Songxin Medical Equipment Co., Ltd | 18cm | |
| URGO silk tape | URGO | 1.25cm × 9.1m |
Odniesienia
- Kong, G., Huang, Z., Zhu, Q., Wan, Y. Comparison of two modified methods of intrathecal catheterization in rats. Exp Anim. 69 (2), 219-223 (2020).
- Xu, C. S., Sun, P., Lin, C. a new design puncture needle and a device of microcatheter protection for lumbar intrathecal catheterization in rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 36 (3), 283-288 (2020).
- Yamamoto, G., et al. Neurosteroid dehydroepiandrosterone sulphate enhances pain transmission in rat spinal cord dorsal horn. Br J Anaesth. 123 (2), e215-e225 (2019).
- Mattioli, T. A., Sutak, M., Milne, B., Jhamandas, K., Cahill, C. M. Intrathecal catheterization influences tolerance to chronic morphine in rats. Anesth Analg. 114 (3), 690-693 (2012).
- Wang, B. C., Hillman, D. E., Li, D., Turndorf, H. Lumbar subarachnoid catheterization in rats. Pharmacol Biochem Behav. 38 (3), 685-688 (1991).
- Yaksh, T. L., Rudy, T. A. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 17 (6), 1031-1036 (1976).
- Martin, H., Kocher, L., Chery-Croze, S. Chronic lumbar intrathecal catheterization in the rat with reduced-length spinal compression. Physiol Behav. 33 (1), 159-161 (1984).
- Mazur, C., et al. Development of a simple, rapid, and robust intrathecal catheterization method in the rat. J Neurosci Methods. 280, 36-46 (2017).
- Zhang, S. X., Huang, F., Gates, M., White, J., Holmberg, E. G. Extensive scarring induced by chronic intrathecal tubing augmented cord tissue damage and worsened functional recovery after rat spinal cord injury. J Neurosci Methods. 191 (2), 201-207 (2010).
- Ohara, P. T. Long-term intrathecal catheterization in the rat. J Neurosci Methods. 110 (1-2), 81-89 (2001).
- StØrkson, R. V., KjØrsvik, A., TjØlsen, A., Hole, K. Lumbar catheterization of the spinal subarachnoid space in the rat. J Neurosci Methods. 65 (2), 167-172 (1996).
- Poon, Y. Y., Chang, A. Y., Ko, S. F., Chan, S. H. An improved procedure for catheterization of the thoracic spinal subarachnoid space in the rat. Anesth Analg. 101 (1), table of contents 155-160 (2005).
- Xu, F., Li, T., Zhang, B. An improved method for protecting and fixing the lumbar catheters placed in the spinal subarachnoid space of rats. J Neurosci Methods. 183 (2), 114-118 (2009).
- Igawa, Y., Andersson, K. E., Post, C., Uvelius, B., Mattiasson, A. A rat model for investigation of spinal mechanisms in detrusor instability associated with infravesical outflow obstruction. Urol Res. 21 (4), 239-244 (1993).
- Hou, Y., et al. A modified procedure for lumbar intrathecal catheterization in rats. Neurol Res. 38 (8), 725-732 (2016).
- Farhadi, E., et al. Comparison of open and ultrasound-guided placement of central venous catheter in children weighing less than five kilograms; a randomized clinical trial. Acad Radiol. 30 (7), 1419-1425 (2023).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone