A subscription to JoVE is required to view this content. Sign in or start your free trial.
Preparing an Antigen-Adjuvant Emulsion to Induce Autoimmune Encephalomyelitis
In This Article
Overview
This video demonstrates an efficient homogenization technique to generate an antigen-adjuvant emulsion to induce experimental autoimmune encephalomyelitis (EAE) in a murine model. The emulsion contains an autoantigen — myelin oligodendrocyte glycoprotein (MOG) — in the water phase, surrounded by a continuous oil phase comprising an inactivated pathogenic bacteria. The emulsion ensures a sustained release of antigens for a prolonged immune response.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
NOTE: A schematic flow of the method is described in Figure 1.
1. Material preparation
NOTE: Prepare all the reagents aseptically in a sterile hood, and aliquot and store at the indicated temperature. The reagents can be stored for up to 2 years without losing their effect.
- Prepare CFA containing 20 mg/mL Mycobacterium tuberculosis as described below.
- Add 100 mg of freeze-dried M. tuberculosis H37RA (see Table of Materials) and five 3.2 mm steel beads to a 7 mL tube (see Table of Materials). Shake the tube in a homogenizer (see Table of Materials) for 60 s at the highest speed setting (5,000 rpm).
- Add 5 mL of incomplete Freund's adjuvant (IFA) to the tube and shake again for 60 s at the highest speed. Transfer the slurry of homogenized M. tuberculosis in IFA (now named CFA) to a fresh tube with a pipette, leaving the beads behind, and store at 4 °C until use.
- Add ultrapure water to the freeze-dried powder of MOG35-55 peptide (see Table of Materials) to obtain a final peptide concentration of 10 mg/mL. Sterilize the solution by passing it through a 0.2 µm filter. Prepare 60 µL aliquots and store at -20 °C until use.
NOTE: The MOG35-55 peptide used for this experiment is of ImmunoGrade purity (~70%), is TFA-cleaved, dissolves easily in water, and contains a C-terminal amide (-NH2) to increase in vivo stability. Peptide aliquots were never thawed and re-frozen more than twice, and were stored at -20 °C until use.
2. Preparation of CFA/peptide emulsions
- Calculate the amount of emulsion needed for immunization. In this standard protocol, each mouse receives 50 µg of MOG35-55 peptide and 300 µg of M. tuberculosis dispersed in Freund's adjuvant (CFA). The amount of each reagent (MOG35-55 peptide, PBS, CFA, and IFA) required for preparing the emulsions can be calculated using Supplemental File 1. An additional 20% of all reagents, to compensate for the small loss of emulsion, is included in the calculation.
NOTE: The commercial emulsion kit (see Table of Materials) used in this study comprises a tube, screw cap, and a plunger in a sterilized pouch. Each tube of the emulsion kit holds a maximum of 9.6 mL, making it possible to prepare 8 x 1 mL syringes sufficient for immunizing 40 mice (or 80 mice if using 100 µL of emulsion per animal). - Place the screw-capped tube (from the commercial emulsion kit) and reagents to be used on ice.
- Add the emulsion components in the following order in the volumes calculated in step 2.1: PBS, then the peptide, then M. tuberculosis in CFA, and finally IFA.
- Close the tube with the cap firmly by tightening and loosening it several times. Shake the tube vigorously for 5-10 s by hand to pre-mix the reagents.
- Place the tube in the shaking homogenizer and secure it with the rod. Set the speed to the highest setting and the time to 60 s. Once the run is finished, place the tube on ice for 3 min. Repeat the run one or two more times with the same settings.
- Centrifuge the tube at 300 x g for 1 min to remove trapped air and compact the emulsion.
- Remove the cap from the tube, insert the plunger into the tube, and push it down slowly until it reaches the top of the emulsion. Remove the snap-off closure at the bottom of the tube by twisting it.
- Remove the plunger from an injection syringe (1 mL; see Table of Materials). Add a needle (preferably 25-27 G) to the injection syringe.
- Attach the back end of the injection syringe to the dedicated lock at the bottom of the tube and lock it with a short twist.
- Transfer the emulsion from the tube to the injection syringe by pushing the plunger gently. Stop when the emulsion reaches the 0.15 mL graduation of the injection syringe.
- Separate the injection syringe from the tube and insert the plunger carefully, taking care that no air enters the syringe. Push the plunger until the emulsion comes out of the needle.
NOTE: At this point, some of the emulsion can be used for quality control (see step 3). - Repeat steps 2.8-2.11 for the rest of the emulsion present in the tube.
NOTE: To minimize the waste of expensive CFA/antigen emulsions, 1 mL syringes should be used. Other syringes that fit the dedicated lock at the bottom of the tube may be used; however, a larger volume of emulsion may need to be prepared. The CFA/MOG35-55 peptide emulsion can be stored at 4 °C for up to 15 weeks without losing its EAE-inducing capacity.
3. Quality control of emulsion
- Drop-test: Add a small drop of the emulsion into a sterile 50 mL conical tube filled with 20 mL of cold water. Close the tube and shake it by hand for a couple of seconds. The appearance of tiny distinct droplets confirms the formation of a water-in-oil emulsion.
- Examine the emulsion using phase contrast microscopy.
- To analyze the size of the water-in-oil particles, add a tiny drop (2-3 µL) of the emulsion to a microscope slide, smear it out with a cover slip, and then push hard in a circular motion to flatten out the emulsion.
- Examine the smeared emulsion under a phase contrast microscope (see Table of Materials) with 400x magnification and focus on a field with a monolayer of the emulsion. Ensure that small uniform grey/white particles are visible (Figure 2A).
Results
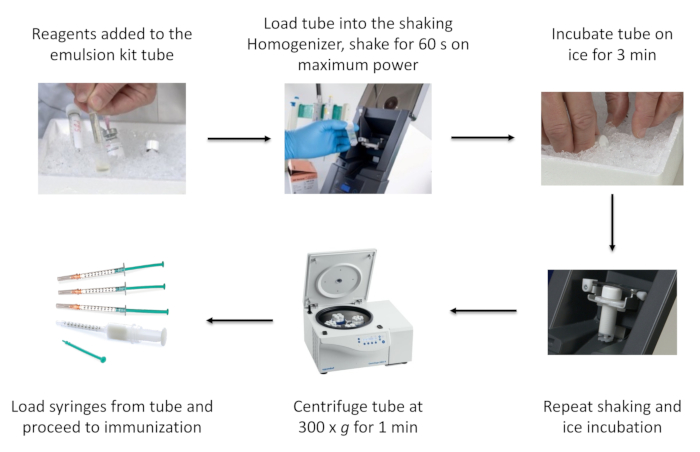
Figure 1: Schematic of the process of emulsion preparation using a commercial emulsion kit. This figure has been reused from Topping et al with permission.
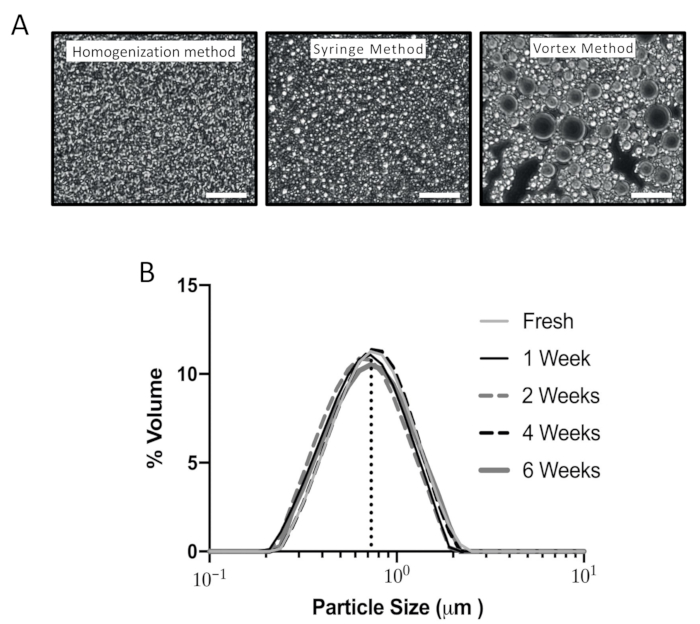
Figure 2: Characterization of emulsions produced using different methods. (A) Representative images from three different preparation meth...
Disclosures
Materials
| Name | Company | Catalog Number | Comments |
| 1 mL Injection syringe | B. Braun | 9166017V | |
| 1 mL Injection syringe | Sigma-Aldrich | Z683531 | |
| 7 ml empty tubes with caps | Bertin-Instruments | P000944LYSK0A.0 | 7 mL tube |
| 50 mL sterile centrifuge tube | Fisher Scientific | 10788561 | 50 mL tube |
| Dispersant, light mineral oil | Sigma-Aldrich | M8410 | Store at RT |
| Emulsion kit | Bertin-Instruments | D34200.10 ea | Containing a tube, cap, and plunger |
| Incomplete Freund's Adjuvant | Sigma-Aldrich | F5506 | Store at +4 °C |
| Mycobacterium tuberculosis, H37RA | Fisher Scientific | DF3114-33-8 | Store at +4 °C |
| Minilys-Personal homogenizer | Bertin-Instruments | P000673-MLYS0-A | Shaking homogenizer |
| MOG35-55 Peptide | Innovagen | N/A | |
| Montanide ISA 51 VG | Seppic | 36362Z | FDA-approved oil adjuvant |
| Pall Acrodisc Syringe Filters 0.2 μm | Fisher Scientific | 17124381 | Sterile filter |
| PBS, Ca2+/Mg2+ free | Thermo Fisher Scientific | 14190144 | PBS |
| Phase-Contrast Microscope | Olympus | BX40-B | |
| Steel Beads 3.2 mm | Fisher Scientific | NC0445832 | Autoclave and store at RT |
This article has been published
Video Coming Soon
Source: Bäckström, B. T. A Rapid, Simple, and Standardized Homogenization Method to Prepare Antigen/Adjuvant Emulsions for Inducing Experimental Autoimmune Encephalomyelitis. J. Vis. Exp. (2022)
Copyright © 2025 MyJoVE Corporation. All rights reserved