A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
A Mouse Model of in Utero Transplantation
In This Article
Summary
The mouse model of in utero transplantation is a versatile tool that can be used to study the potential clinical applications of stem cell transplantation and gene therapy in the fetus. In this protocol, we present a general approach to performing this technique
Abstract
The transplantation of stem cells and viruses in utero has tremendous potential for treating congenital disorders in the human fetus. For example, in utero transplantation (IUT) of hematopoietic stem cells has been used to successfully treat patients with severe combined immunodeficiency.1,2 In several other conditions, however, IUT has been attempted without success.3 Given these mixed results, the availability of an efficient non-human model to study the biological sequelae of stem cell transplantation and gene therapy is critical to advance this field. We and others have used the mouse model of IUT to study factors affecting successful engraftment of in utero transplanted hematopoietic stem cells in both wild-type mice4-7 and those with genetic diseases.8,9 The fetal environment also offers considerable advantages for the success of in utero gene therapy. For example, the delivery of adenoviral10, adeno-associated viral10, retroviral11, and lentiviral vectors12,13 into the fetus has resulted in the transduction of multiple organs distant from the site of injection with long-term gene expression. in utero gene therapy may therefore be considered as a possible treatment strategy for single gene disorders such as muscular dystrophy or cystic fibrosis. Another potential advantage of IUT is the ability to induce immune tolerance to a specific antigen. As seen in mice with hemophilia, the introduction of Factor IX early in development results in tolerance to this protein.14
In addition to its use in investigating potential human therapies, the mouse model of IUT can be a powerful tool to study basic questions in developmental and stem cell biology. For example, one can deliver various small molecules to induce or inhibit specific gene expression at defined gestational stages and manipulate developmental pathways. The impact of these alterations can be assessed at various timepoints after the initial transplantation. Furthermore, one can transplant pluripotent or lineage specific progenitor cells into the fetal environment to study stem cell differentiation in a non-irradiated and unperturbed host environment.
The mouse model of IUT has already provided numerous insights within the fields of immunology, and developmental and stem cell biology. In this video-based protocol, we describe a step-by-step approach to performing IUT in mouse fetuses and outline the critical steps and potential pitfalls of this technique.
Protocol
1. Preparation of Injection Pipettes
- Calibrate the pipette puller such that separation of the glass pipette occurs within 15 seconds (see manufacturer's instructions regarding calibration). The pipette will have a taper where it separates.
- Cut the end of the pipette such that the distance from the beginning of the taper to the end of the pipette is 1.04cm to 1.05cm. The length of the pipette is inversely proportional to the caliber of the pipette orifice. Be aware that making a longer pipette also results in a weaker tip that is more susceptible to breakage during the injection.
- The next step in making the pipette is to create a smooth bevel by sharpening the tip on a diamond sharpening wheel. At the time of cutting the pipette to the appropriate size, the pipette often breaks with a natural bevel at its tip which must be used when placing the pipette on the wheel. During this process, it is important to gently rest the pipette on the sharpening wheel and periodically re-evaluate to make sure the pipette is still touching the wheel (as the bevel becomes smoother, it will lose contact with the sharpening wheel).
- Once the bevel is smooth, the pipette tip must be sharpened (Figure 1). Elevate the pipette such that it is no longer touching the sharpening wheel, rotate it clockwise by 10-50 degrees, and place the pipette back on the rotating sharpening wheel for ~10 seconds. Withdraw the pipette from the sharpening wheel and now examine the edge. This step usually needs to be repeated with several small adjustments to create a sharp edge. If the pipette is placed on its edge for too long, it is more likely to develop an uneven surface (Figure 1). After completing one side, you can proceed to sharpen the opposite edge by repeating the steps above.
- Use a permanent marker to draw a circumferential line every 4mm starting where the taper of the pipette begins. These demarcations correspond to 5uL of volume.
2. in Utero Transplantation
- After preparing the injectable material (e.g. cells, virus, etc.) you can set up for the injection. We use a microinjector connected to compressed air with the following settings: (Pressure settings: input 60-80 psi, fill 40 psi, inject 8-12 psi, balance 0 psi, hold 0 psi; Injection time 100 milliseconds).
- To sterilize the injection pipette, clean it twice with 70% EtOH followed by twice with sterile 1X PBS. As the tip of the pipette is very fragile, we do not recommend autoclaving the injection pipettes.
- Injections can either be done using 2.5X-3.5X magnification loupes or a dissecting microscope. Prepare your procedure area with a warming blanket, lighting, and necessary surgical instruments.
- Anesthetize the pregnant mouse (we utilize isoflurane and oxygen delivered via a continuous flow anesthesia unit). Place the mouse on a heating pad in the supine position and affix each limb with tape to secure the mouse in place.
- Clip the fur. Then, wearing sterile surgical gloves, prep the abdomen with 10% povidone-iodine followed by alcohol, and inject an analgesic such as buprenorphine.
- Make a 1cm incision in the lower abdomen (the most inferior aspect of the incision should be approximately 1cm superior to the introitus). Incise the skin and the fascia. Be careful not to injure the abdominal organs (intestines and bladder) which are immediately under the thin layer of fascia.
- Using cotton swabs, gently stretch the fascia and deliver the gravid uterus through the incision. Count the total number of fetuses by first identifying the right and left ovaries to make sure you visualize the entire uterus. Place the uterus back in the abdominal cavity before you proceed so that the fetuses remain warm while you prepare for the injections.
- Draw up the appropriate volume of material for the number of fetuses you plan to inject. While filling the needle with your sample, it is important to keep the pipette tip submersed to avoid aspirating your sample into the microinjector tubing. Since cells are generally in a small volume, we usually place them in a small microfuge (ex: 0.5mL) tube or directly on a piece of Parafilm to be able to fill the needle without breaking the pipette tip.
- Gently hold each fetus and identify the part of the fetus you plan to inject (e.g. liver, peritoneal cavity, leg, etc). With your thumb and forefinger, you must stabilize the fetus within the amniotic cavity so that it does not rotate while you are performing the injection. It is important to hold the fetus firmly enough to stabilize it yet gently enough to avoid damaging it.
- Carefully insert the pipette through the uterus, amnion, and fetal skin, and into the target organ. If the pipette is sharp, it should pass easily through these tissues. If the pipette tip is dull, you will notice tenting of the uterus and amniotic membranes. Inject the desired volume into each fetus. It is imperative that your movements be steady during the time of the insertion, injection, and withdrawal of the pipette.
- Once the desired volume is injected, withdraw the pipette carefully. If done properly, material should not leak out from the amniotic cavity or uterus. If a large hole is created in the amnion, which one can recognize by leakage of amniotic fluid, then survival is jeopardized. Finally, for studies that involve postnatal harvesting, all fetuses must receive technically perfect injections since there is no way to discern injected and uninjected fetuses after birth.
- If you are performing injections into the fetal liver, you may occasionally see blood in the pipette tip after it is withdrawn- this confirms you are in the correct position and should not affect survival. If performing intra-peritoneal injections, aim slightly below the fetal liver. For intramuscular injections, you may position the fetus to identify the hip and femur and inject the gluteus muscle. Finally, for intraventricular injections, it is easy to see the coronal sutures to direct the pipette to the appropriate target. For these injections, slightly larger caliber pipettes are required to penetrate the skull without breaking the pipette.
- Next, carefully place the uterus back into the abdominal cavity. Make sure it is not twisted on itself or around its vascular supply. Deliver 1mL of sterile 1X PBS in the mother's peritoneal cavity to replace any fluid that was lost during the procedure. Close the incision in 2 layers with a 5-0 braided absorbable suture (ex: Vicryl). First close the fascia without injuring the underlying bowel or bladder, and then close the skin.
- At this time, post-procedure medications can be administered for analgesia. Return each injected female to its own individual cage and place the cage on a warming blanket. Monitor the mouse until it is upright. Put bedding in the cage and place the mouse in a quiet area of the mouse facility where it should not be disturbed (i.e. no cage cleaning) until several days after delivery. Minimizing any additional stress for the animal will decrease the chances of preterm labor.
- Once you have completed the injections, clean your pipette twice with sterile 1X PBS and once with 70% EtOH.
- Injected females should be observed daily to insure they are recovering from the procedure without difficulty. The incision should be monitored for swelling, inflammation, wound separation, and other signs of infection. Post-operative analgesics can be administered as needed.
3. Representative Results:
Survival of the injected fetuses to term delivery is the main limiting factor to achieving success with this technique. Depending on the material injected and the strain of mouse being used, survival rates can vary. In general, injections of hematopoietic cells into wild-type mice at E14 should result in at least 50% live-born pups. Higher rates of survival are possible depending on both the technical aspects of the injection as well as the characteristics of the mice being injected.
Minimizing trauma to the uterus and amniotic membranes is the most important technical aspect of this protocol. Sharp, small caliber pipettes will result in minimal uterine trauma during the injection. We do not recommend using standard injection needles as the caliber of pre-fabricated needles is too large and would result in a large hole in the uterus. Hand crafted glass injection pipettes are the only small caliber needles that can be utilized for in utero injections. Careful and meticulous surgical technique, including gentle handling of the uterus and a short anesthetic is also crucial for optimal results.
Specific characteristics of the recipient mice such as the genetic background, gestational age, and litter size can also affect survival. Certain strains of mice are more susceptible to preterm labor and pregnancy loss depending on their genetic background.15 Transgenic animals with muscular or neurodegenerative defects can have an impaired ability to deliver fetuses vaginally after undergoing a midline laparotomy.8 These pregnant females may require delivery by cesarean section. We have also found that the gestational age at the time of injection can impact viability. Fetuses that are younger than embryonic day 12 have lower rates of survival than older fetuses. Finally, we have found that large litter sizes (>10 fetuses) tend to have higher rates of fetal demise after injection. Attention to both the technical aspects of this technique and the specific characteristics of the mice being injected can maximize survival of the injected fetuses.
When these methods are performed correctly, one can expect that all fetuses are exposed to the injected material. Similar to the postnatal setting, however, the successful delivery of cells or viruses in utero does not always result in donor cell engraftment or gene expression, respectively. The engraftment of stem cells, for example, is dependent on several factors such as the dose and source of the transplanted cells. Similarly, the success of viral transduction is, in part, determined by the type of viral vector used. One must understand the numerous factors that impact pup survival, cellular engraftment, and viral transduction to achieve success with this protocol.
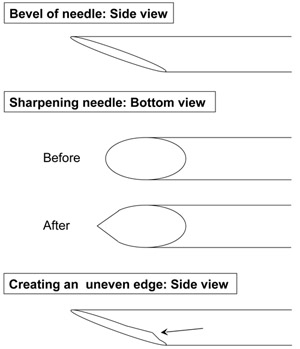
Figure 1. Diagram depicting proper sharpening of the injection pipettes (Step 1.4)
Discussion
Over 50 years ago, Billingham, Brent, and Medawar used in utero transplantation in mice to induce immune tolerance to foreign proteins.16 Since that time, several variations of this technique have been used to address questions in immunology and stem cell biology.
The protocol detailed here is one of the most accessible methods for IUT. The fetal liver offers an easily visualized target and provides access to the systemic circulation via the portal and hepatic veins. Ho...
Disclosures
No conflicts of interest declared.
Acknowledgements
We would like to acknowledge our funding sources: The California Institute for Regenerative Medicine Clinical Fellow Training Grant (AN), National Science Foundation (MW), Irene Perstein Award (TCM), American College of Surgeons (TCM), American Pediatric Surgical Association (TCM), and the March of Dimes (TCM).
Materials
| Name | Company | Catalog Number | Comments |
| Pipettes | Kimble Chase | 71900-100 | |
| Pipette puller | Sutter Instrument Co. | Model P-30 | |
| Microinjector | Narishige International | IM-300 | |
| Pipette sharpener | Sutter Instrument Co. | Model BV-10 |
References
- Flake, A. W. Treatment of X-linked severe combined immunodeficiency by in utero transplantation of paternal bone marrow. N Engl J Med. 335, 1806-1810 (1996).
- Wengler, G. S. In-utero transplantation of parental CD34 haematopoietic progenitor cells in a patient with X-linked severe combined immunodeficiency (SCIDXI). Lancet. 348, 1484-1487 (1996).
- Flake, A. W., Zanjani, E. D. in utero hematopoietic stem cell transplantation: ontogenic opportunities and biologic barriers. Blood. 94, 2179-2191 (1999).
- Merianos, D. J. Maternal alloantibodies induce a postnatal immune response that limits engraftment following in utero hematopoietic cell transplantation in mice. J Clin Invest. 119, 2590-2600 (2009).
- Peranteau, W. H., Endo, M., Adibe, O. O., Flake, A. W. Evidence for an immune barrier after in utero hematopoietic-cell transplantation. Blood. 109, 1331-1333 (2007).
- Kim, H. B., Shaaban, A. F., Yang, E. Y., Liechty, K. W., Flake, A. W. Microchimerism and tolerance after in utero bone marrow transplantation in mice. J Surg Res. 77, 1-5 (1998).
- Durkin, E. T., Jones, K. A., Rajesh, D., Shaaban, A. F. Early chimerism threshold predicts sustained engraftment and NK-cell tolerance in prenatal allogeneic chimeras. Blood. 112, 5245-5253 (2008).
- Mackenzie, T. C., Shaaban, A. F., Radu, A., Flake, A. W. Engraftment of bone marrow and fetal liver cells after in utero transplantation in MDX mice. J Pediatr Surg. 37, 1058-1064 (2002).
- Hayashi, S. Mixed chimerism following in utero hematopoietic stem cell transplantation in murine models of hemoglobinopathy. Exp Hematol. 31, 176-184 (2003).
- Bouchard, S. Long-term transgene expression in cardiac and skeletal muscle following fetal administration of adenoviral or adeno-associated viral vectors in mice. J Gene Med. 5, 941-950 (2003).
- Meza, N. W. Rescue of pyruvate kinase deficiency in mice by gene therapy using the human isoenzyme. Mol Ther. 17, 2000-2009 (2009).
- MacKenzie, T. C. Efficient transduction of liver and muscle after in utero injection of lentiviral vectors with different pseudotypes. Mol Ther. 6, 349-358 (2002).
- MacKenzie, T. C. Transduction of satellite cells after prenatal intramuscular administration of lentiviral vectors. J Gene Med. 7, 50-58 (2005).
- Sabatino, D. E. Persistent expression of hF.IX After tolerance induction by in utero or neonatal administration of AAV-1-F.IX in hemophilia B mice. Mol Ther. 15, 1677-1685 (2007).
- Mellor, A. L., Munn, D. H. Immunology at the maternal-fetal interface: lessons for T cell tolerance and suppression. Annu Rev Immunol. 18, 367-391 (2000).
- Billingham, R. E., Brent, L., Medawar, P. B. Actively acquired tolerance of foreign cells. Nature. 172, 603-606 (1953).
- Endo, M. Gene transfer to ocular stem cells by early gestational intraamniotic injection of lentiviral vector. Mol Ther. 15, 579-587 (2007).
- Waddington, S. N. Long-term transgene expression by administration of a lentivirus-based vector to the fetal circulation of immuno-competent mice. Gene Ther. 10, 1234-1240 (2003).
- Schachtner, S., Buck, C., Bergelson, J., Baldwin, H. Temporally regulated expression patterns following in utero adenovirus-mediated gene transfer. Gene Ther. 6, 1249-1257 (1999).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved