A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Acute Myocardial Infarction in Rats
In This Article
Summary
The rat model of acute myocardial infarction (AMI) is useful to study the consequence of a MI on cardiac pathophysiological and physiological function.
Abstract
With heart failure leading the cause of death in the USA (Hunt), biomedical research is fundamental to advance medical treatments for cardiovascular diseases. Animal models that mimic human cardiac disease, such as myocardial infarction (MI) and ischemia-reperfusion (IR) that induces heart failure as well as pressure-overload (transverse aortic constriction) that induces cardiac hypertrophy and heart failure (Goldman and Tarnavski), are useful models to study cardiovascular disease. In particular, myocardial ischemia (MI) is a leading cause for cardiovascular morbidity and mortality despite controlling certain risk factors such as arteriosclerosis and treatments via surgical intervention (Thygesen). Furthermore, an acute loss of the myocardium following myocardial ischemia (MI) results in increased loading conditions that induces ventricular remodeling of the infarcted border zone and the remote non-infarcted myocardium. Myocyte apoptosis, necrosis and the resultant increased hemodynamic load activate multiple biochemical intracellular signaling that initiates LV dilatation, hypertrophy, ventricular shape distortion, and collagen scar formation. This pathological remodeling and failure to normalize the increased wall stresses results in progressive dilatation, recruitment of the border zone myocardium into the scar, and eventually deterioration in myocardial contractile function (i.e. heart failure). The progression of LV dysfunction and heart failure in rats is similar to that observed in patients who sustain a large myocardial infarction, survive and subsequently develops heart failure (Goldman). The acute myocardial infarction (AMI) model in rats has been used to mimic human cardiovascular disease; specifically used to study cardiac signaling mechanisms associated with heart failure as well as to assess the contribution of therapeutic strategies for the treatment of heart failure. The method described in this report is the rat model of acute myocardial infarction (AMI). This model is also referred to as an acute ischemic cardiomyopathy or ischemia followed by reperfusion (IR); which is induced by an acute 30-minute period of ischemia by ligation of the left anterior descending artery (LAD) followed by reperfusion of the tissue by releasing the LAD ligation (Vasilyev and McConnell). This protocol will focus on assessment of the infarct size and the area-at-risk (AAR) by Evan's blue dye and triphenyl tetrazolium chloride (TTC) following 4-hours of reperfusion; additional comments toward the evaluation of cardiac function and remodeling by modifying the duration of reperfusion, is also presented. Overall, this AMI rat animal model is useful for studying the consequence of a myocardial infarction on cardiac pathophysiological and physiological function.
Protocol
1. Preparation of the Operating Area
- The operating surface is first prepared by disinfecting the area with 70% ethanol.
- All surgical instruments are to be sterilized with a hot bead sterilizer before surgery (and in between individual rat surgeries). These instruments include: surgical scissors (2), forceps (1), curved forceps (1), needle holder (2), and a chest rectractor.
- Cotton gauze and applicators are to be available in order to manage bleeding.
- Adjust the temperature of a homeothermic blanket system (heating pad), to be used to maintain the body temperature of the animal at 37±1°C, in order to prevent a dramatic fall in body temperature; the size of an infarct is dependent on the duration of occlusion as well body temperature (Tarnavski et al.).
- Prior to anesthesia, each animal will receive a dosage of buprenorphine (0.1-2.5 mg/kg SQ) and carprofen oral tablets (Rimadyl, Bioserv tablet; 1 tablet) the day before the surgery. At the time immediately prior to the surgery, each animal will again receive a dosage of buprenorphine (0.1-2.5 mg/kg SQ).
- Note: For studies involving the assessment of cardiac function and cardiac remodeling over a period of time (7- to 28-days and beyond), a dosage of buprenorphine (0.1-2.5 mg/kg SQ) will be administered, every 6-hours after surgery for 24-48 hours post surgery and carprofen oral tablets will be provided once daily (see "Post-Operative Recovery").
2. Preparation and Intubation of Rats
- Prior to the induction of anesthesia, animals will be examined to identify any pre-existing conditions that may complicate surgical outcome; physical examination will include visual inspection and measurement of heart rate, respiratory rate, and body temperature and body weight.
- If the animals are determined to be healthy, 250-300 gram male rats (e.g. Sprague Dawley) will be weighed and identifying characteristics will be recorded.
- Rats are then anesthetized with 80 mg/kg of pentobarbital; the correct level of anesthesia is verified through the limb withdrawal response by applying pressure of the rat nail bed (toe-pinch reflex) and periodically throughout the procedure.
- Buprenorphine (0.1-2.5 mg/kg; SQ) will be administered at the time of surgery.
- An electric shaver is then used to shave the fur form the neck and chest areas. Note: For studies involving the assessment of cardiac function and cardiac remodeling over a period of time (7- to 28-days and beyond), fur must be completely shaved away from the surgical area to maintain sterility.
- The shaved areas are to be scrubbed and disinfected with betadine solution followed by wiping the area with 70% alcohol; this betadine and alcohol scrub and disinfection procedure is to be repeated three times.
- The anesthetized animal is then placed in a supine position on a homeothermic blanket system (heating pad) in order to maintain body temperature.
- Endotracheal intubation is then performed by first carefully exposing the trachea, to enhance visualization of intubation, by making a 5 mm mid-neck incision and retraction of muscle tissue, just above the trachea.
- This allows better visualization for insertion of the endotracheal tube (polyethylene size 90; PE 90) where the tubing is beveled on the edge for ease of entrance through the larynx.
- The endotracheal tube is inserted into the trachea, with visualization aided by using a surgical stereo microscope, by carefully manipulating the tongue while viewing the trachea and the entrance of the endotracheal tube.
- Mechanical ventilation is then achieved by connecting the endotracheal tube to Kent Scientific ventilator (TOPO Dual Mode) cycling at 80 breaths per minute and a tidal volume of 1.2 ml per 100 gram body weight.
- A sterile drape is then placed over the rat, leaving only the surgical area exposed, thus minimizing potential contamination during the operation.
- The animal surgeon and assistants are to wear mask, gown, and gloves; with gloves being changed between individual rat surgeries.
3. Transient Left Anterior Descending (LAD) Artery Ligation
- Once steady breathing is established, the chest is opened at the left fourth intercostal space, by using hemostats or round end scissors to open the space, but without cutting the tissue so that the risk of bleeding can be reduced.
- A chest retractor is then positioned within the fourth intercostal space; in order to spread the ribs so that the left ventricle (LV) is exposed, taking maximal care not to damage the lung.
- The pericardium is then opened, but left in place if possible.
- The proximal left anterior descending (LAD) artery will be identified (Figure-1), with the use of a surgical stereo microscope, and the LAD is then transiently ligated (or can be tied with a slipknot) using a 6-0 prolene suture for a 30-minute ischemic period (Figure-1), but without exteriorization of the heart.
- To allow cardiac reperfusion, microsurgical scissors are used to cut the knot in the ligature (or by releasing the slipknot), made by the 6-0 prolene suture, 30-minutes following ligation of the LAD.
- In sham control rats, the procedure is identical, except the LAD is not transiently ligated.
- The outflow is then briefly (1-2 seconds) pinched off on the respirator to allow re-inflation of the lungs.
- The chest retractor is then removed and the ribs are drawn together using a 2-0 prolene suture with an interrupted suture pattern.
- Once the ribs are closed, the outflow of the ventilator is again briefly (1-2 seconds) pinched off to ensure proper breathing.
- The skin is closed using 6-0 prolene suture with a continuous suture pattern.
4. Confirmation of Successful Ligation of the LAD Artery
- Proper ligation of the LAD will be confirmed by observing blanching of myocardial tissue distal to the suture as well as dysfunction of the anterior wall; as observed during the transient LAD ligation.
- Reperfusion will be verified by the return of red color to the myocardial tissue and the demonstration of some recovery of anterior wall motion; as observed immediately following the transient LAD ligation.
- Note: For studies involving the assessment of cardiac function and cardiac remodeling over a period of time (7- to 28-days and beyond), an echocardiogram will be performed 24-hours post LAD ligation, in order to determine the extent of the induced myocardial infarction. All animals with a left ventricular fractional shortening (LV FS) < 20%, as determined from an echocardiogram, as well as anterior wall motion dysfunction consistent with the infarct, will be identified and used for follow-up cardiac function and remodeling studies. Sham control rats are expected to have a normal LV FS between 40-45% (del Monte et al).
5. Post-Operative Recovery
- Once the surgery is complete, the sterile drape over the animal will be removed. The body temperature (by a rectal thermometer), respiratory rate (by visual inspection), heart rate (by palpitation), and abnormal signs of pain are to be monitored until the animal becomes ambulatory.
- The rat will be removed from the ventilator and then be placed in an animal cage and allowed to recover from anesthesia. In this cage, one end of the cage will be warmed by a heating pad to allow the rat to seek warmth. Also, water will be available to the rat during this observation period.
- If signs of dehydration are observed after surgery, 0.5 ml of sterile saline will be injected intraperitoneally (IP).
- Buprenorphine (0.1‐2.5 mg/kg; SQ) will then be administered immediately after surgery, and during post-operative recovery.
- Note: For studies involving the assessment of cardiac function and cardiac remodeling over a period of time (7- to 28-days and beyond), a dosage of buprenorphine (0.1-2.5 mg/kg SQ) will be administered, every 6-hours after surgery for 24-48 hours post surgery. Carprofen oral tablets (Rimadyl, Bioserv tablet) will also be provided (1-tablet daily) for at least 48-hours post surgery.
6. Histological Evaluation of the Ischemia Reperfusion
- Following completion of the 4-hour reperfusion period, rats are to be anesthetized again with 80 mg/kg of pentobarbital and the LAD is ligated once again (or the slipknot can be retied) (Huang et al and Kaiser et al).
- 1 ml of 5 % Evan's Blue (in phosphate-buffered saline) is then injected into the right ventricular chamber and allowed to perfuse for 2-minutes.
- After 2-minutes, 1 ml of saturated potassium chloride solution is then injected into the right ventricular chamber in order to stop the heart from beating.
- The heart is then rapidly excised, embedded in 2% agarose phosphate-buffered saline, and then sectioned in 1-2 mm slices.
- The viable myocardium is then stained with 2% triphenyl tetrazolium chloride (TTC) for 20-minutes and images of each slice is captured. The images are then quantified in order to identify the amount of myocardial area: (1) that is not-at-risk (blue sections), (2) area-at-risk (AAR; red and white sections), and (3) area that is infarcted (white "unstained" sections) (Figure-2).
7. Representative Results
For studies involving the assessment of infarct size and the area-at-risk (AAR), by Evan's blue dye and triphenyl tetrazolium chloride (TTC), following 4-hours of reperfusion, proper ligation of the LAD can be confirmed by observing blanching of myocardial tissue distal to the suture as well as dysfunction of the anterior wall; as observed during the transient LAD ligation. Reperfusion can be verified by the return of red color to the myocardial tissue and the demonstration of some recovery of anterior wall motion; observed immediately following the transient LAD ligation. For studies involving the assessment of cardiac function and cardiac remodeling over a period of time (7- to 28- days and beyond), proper ligation of the ischemia followed by reperfusion can also be confirmed by blanching of the tissue, dysfunction of the anterior wall and the return of red color with some recovery of the wall motion following reperfusion. In addition, impaired cardiac function can be determined by an echocardiogram being performed 24-hours post LAD ligation, in order to determine the extent of the induced myocardial infarction. All animals with a left ventricular fractional shortening (LV FS) < 20%, as determined from an echocardiogram, as well as anterior wall motion dysfunction consistent with the infarct, will be identified and used for follow-up cardiac function and remodeling studies. Sham control rats are expected to have a normal LV FS between 40-45% (del Monte).
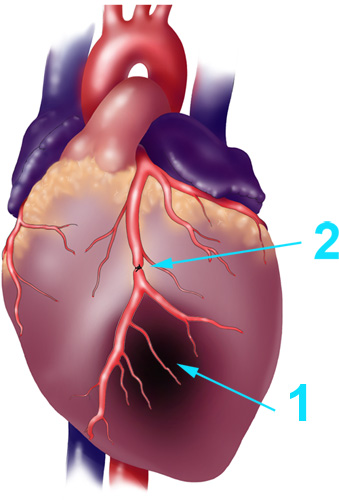
Figure 1. Schematic Diagram of a Myocardial Infarction and LAD Ligation. Schematic diagram of (1) myocardial infarction and (2) ligation of the left ventricular anterior descending (LAD) artery using a suture, used to mimic the myocardial infarction caused by plague formation.

Figure 2. Representative Image of a Rat Heart Following AMI. Representative image of the rat heart showing the area in blue (non-occluded coronary perfusion area), area in red (occluded coronary perfusion area indicating the area-at-risk (AAR) for infarction, and area in white/yellow (infarcted zone within the AAR).
Discussion
An acute myocardial infarction (AMI) in rats, also referred to as ischemia-reperfusion (IR), is commonly used to mimic human ventricular remodeling leading to heart failure. The progression of LV dysfunction and heart failure in animal models (rats and mice) is similar to that observed in humans who sustain a MI, survive and then subsequently develops heart failure (Goldman). This procedure outlined one version of this technique and also contained additional notes describing methodological variations. Additional such var...
Disclosures
No conflicts of interest declared.
Acknowledgements
B.K.M. is supported by a National Institute of Health (NIH) / National Heart Lung and Blood Institute (NHLBI) grant (R01- HL085487). M.H. is supported by the American Heart Association National (AHA) Scientist Development Grant (SDG) and the Society of Geriatric Cardiology.
Materials
| Material Name | Type | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| Name | Company | Catalog Number | Comments | |
| Ventilator | Kent Scientific | TOPO | TOPO Dual Mode | |
| Thermostatic Circulator E103 | Harvard Apparatus | 732802 | ||
| Homeothermic Blanket System | Harvard Apparatus | 55-7222F | ||
| Stereo Microscope | Nikon Instruments | MMA36300 | SMZ-645 Zoom Stereo Microscope | |
| Hot Bead Sterilizer | Harvard Apparatus | 610183 | ||
| Electric Shaver | General Electric | |||
| Blades and Handles | WelchAllyn | 690047-1 | Standard (Lamp) Laryngoscope Blades | |
| Surgical Scissors | Fine Science Tools | 14014-17 | ||
| Surgical Scissors | Fine Science Tools | 14072-10 | ||
| Forceps | Fine Science Tools | 11028-15 | ||
| Curved Forceps | Fine Science Tools | 11001-12 | ||
| Needle Holder | Fine Science Tools | 12004-18 | ||
| Needle Holder | Fine Science Tools | 91201-13 | ||
| Chest Retractor | Fine Science Tools | 17008-07 | ||
| Masks | VWR | 47730-640 | ||
| Gauze Sponges | Dermacea | 441205 | ||
| Sterile Gloves | Cardinal Health | 2D7203I | ||
| PE 90 tubing | BD | 427420 | ||
| 20 ml Syringe | BD | 301625 | ||
| 6-0 Prolene Suture | ETHICON | 8706H | ||
| 2-0 Silk Suture | ETHICON | K873H | ||
| Pentobarbital Sodium Injection | OVATION | NDC 67386-501-55 | ||
| Povidone-Iodine | NOVAPLUS | V10-8204 | ||
| Buprenorphine | Pharmaceuticals Inc. | NDC 12496-0757-1 |
References
- Hunt, S. A., Baker, D. W., Chin, M. H., Cinquegrani, M. P., Feldmanmd, A. M., Francis, G. S., Ganiats, T. G., Goldstein, S., Gregoratos, G., Jessup, M. L., Noble, R. J., Packer, M., Silver, M. A., Stevenson, L. W. SC.ACC/AHA Guidelines for the Evaluation and Management of Chronic Heart Failure in the Adult: Executive Summary A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure). Circulation. 104, 2996-3007 (2001).
- Goldman, S., Raya, T. E. Rat infarct model of myocardial infarction and heart failure. J Card Fail. 1, 169-177 (1995).
- Tarnavski, O., McMullen, J. R., Schinke, M., Nie, Q., Kong, S., &, I. z. u. m. o., S, . Mouse cardiac surgery: comprehensive techniques for the generation of mouse models of human diseases and their application for genomic studies. Physiol Genomics. 16, 349-360 (2004).
- Thygesen, K., Alpert, J. S. Universal Definition of Myocardial Infarction. Circulation. 116, 2634-2653 (2007).
- Vasilyev, N., Williams, T., Brennan, M. L., Unzek, S., Zhou, X., Heinecke, J. W., Spitz, D. R., Topol, E. J., Hazen, S. L., Penn, M. S. Myeloperoxidase-generated oxidants modulate left ventricular remodeling but not infarct size after myocardial infarction. Circulation. 112, 2812-2820 (2005).
- McConnell, B. K., Popovic, Z., Mal, N., Lee, K., Bautista, J., Forudi, F., Schwartzman, R., Jin, J. P., Penn, M., Bond, M. Disruption of protein kinase A interaction with A-kinase-anchoring proteins in the heart in vivo: effects on cardiac contractility, protein kinase A phosphorylation, and troponin I proteolysis. J Biol Chem. 284, 1583-1592 (2009).
- Monte, D. e. l., Butler, F., Boecker, K., Gwathmey, W., K, J., Hajjar, R. J. Novel technique of aortic banding followed by gene transfer during hypertrophy and heart failure. Physiol. Genomics. 9, 49-56 (2002).
- Huang, M. H., Nguyen, V., Wu, Y., Rastogi, S., McConnell, B. K., Wijaya, C., Uretsky, B. F., Poh, K. K., Tan, H. C., Cheng, J., Perin, E., Fujise, K. Synergistic Infarct Size Reduction by Combination Therapy with Milrinone and Esmolol at Early Reperfusion during Acute Myocardial Infarction. American Journal of Physiology. , .
- Kaiser, R. A., Bueno, O. F., Lips, D. J., Doevendans, P. A., Jones, F., Kimball, T. F., Molkentin, J. D. Targeted inhibition of p38 mitogen-activated protein kinase antagonizes cardiac injury and cell death following ischemia-reperfusion in vivo. J Biol Chem. 279, 15524-15530 (2004).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved