A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Collecting Variable-concentration Isothermal Titration Calorimetry Datasets in Order to Determine Binding Mechanisms
In This Article
Summary
ITC is a powerful tool for studying the binding of a ligand to its host. In complex systems however, several models may fit the data equally well. The method described here provides a means to elucidate the appropriate binding model for complex systems and extract the corresponding thermodynamic parameters.
Abstract
Protocol
1. Preparing stock solutions
- Purify the macromolecule of interest. (In this case, aminoglycoside N-6'-acetyltransferase-Ii (AAC6'-Ii), is isolated as reported elsewhere.13)
- Prepare 4 litres of dialysis buffer. (In this case we used 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES, MW 238.3 g/mol), containing 2 mM ethylenediaminetetraacetic acid (EDTA, MW 292.2), at pH 7.5.)
- Dialyze the protein The AAC(6')-Ii sample (5 mL at 400 μM) used must be dialyzed in dialysis buffer (3 x 1.3 L). The final dialysis solution is kept to rinse the machine and to dilute samples.
- Filter the final dialysis solution through a 0.45 μm cellulose filter, and stored at 4°C. Will be referred to as 'running buffer'
- Filter protein solution through a 0.2 μm syringe filter which has been rinsed thoroughly with running buffer. Measure the final concentration of the protein using a standard assay (Bradford, Lowry, etc.) or UV absorbance. Store the protein to maximize long-term stability. (In the case of AAC(6')-Ii, this means storage at 4°C. AAC(6')-Ii does not retain activity after freezing and thawing).
- Prepare 200μL of 25 mM stock solution of acetyl coenzyme A (AcCoA, the ligand, MW 809.57) by dissolving 4.0 mg in running buffer. Freeze at -78°C until ready to use.
- All protein and ligand samples must originate from the same stock solutions, to minimize random sample-to-sample fluctuations in concentration. A single correction factor for the protein concentration across the entire variable-c dataset is adjusted in the analysis.10
2. Preparing ITC samples
- Dilute the enzyme solution to a c-value of 64 with a final volume of 2 mL. (In the case of AAC(6')-Ii, this corresponds to 192 μM.)
- Quickly thaw the ligand (AcCoA) stock solution in ice water.
- Prepare 0.5 mL of a AcCoA solution 10 times more concentrated then the number of binding sites on the protein, in this case 4 mM, by diluting 80 μL of the AcCoA stock solution in 420 μL of running buffer. Transfer into a pipette filling tube. Quickly return the AcCoA stock solution to -78°C.
- Degas the protein and solutions under vacuum for 5 min at a temperature 1°C below the desired running temperature (19 °C).
3. Setting up the syringe14
- Insert the injection syringe through syringe holder until the pre-mounted syringe clamp is at the same height as the holder.
- Feed the second syringe clamp over the injection syringe until it is firmly pressed against the bottom of the syringe holder. Gently tighten the clamp with the provided 0.050" Ball Point Hex Drive.
- Place syringe holder into the pipette holder.
- Gently slide the pipette injector into the injection syringe. Make sure the plunger tip is fed directly into the hole of the syringe. Once fully inserted, screw the locking collar of the syringe holder into the pipette injector.
4. Loading the sample cell14
- Wash sample cell with a minimum of 50 mL of running buffer, and remove any remaining liquid by using a long-needled 2.5 ml glass syringe.
- Slowly draw a minimum of 1.8 ml of protein sample solution into the clean and dry long-needled 2.5 ml syringe. Be careful not to introduce any bubbles.
- Carefully insert needle in sample cell and gently touch the bottom of the cell. Raise tip slightly (~1 mm) and gently inject AAC(6')-Ii solution into the cell until excess liquid is visible above the top of the sample cell.
- Slowly raise the needle about 1 cm while ensuring liquid remains in the overflow. Quickly withdraw and inject a small amount of solution (~0.25 ml) to remove any trapped bubbles in the sample cell.
- Remove all solution overflow. This is achieved by gently sliding the needle along side of overflow into the sample cell. The syringe tip will hit a ledge, this is the desire height for the running solution. Remove all liquid which sits above this line.
5. Loading injection syringe and initiating run14
- Attach the plastic tube loading syringe into fill port.
- Lower plunger tip to the top of the fill port.
- Place the AcCoA solution in the pipette filling tube at the bottom of the pipette holder. The syringe tip should not touch the bottom of the filling tube.
- Slowly draw the solution into the syringe until a small amount enters the tube of the loading syringe.
- Close the fill port by lowering the plunger tip and click on the Close Fill Port button. Purge and refill, by clicking on the Purge->Refill button, 3 times to remove any air bubbles that may have become trapped during loading.
- Remove the filling tube from the pipette holder and gently wipe the syringe tip.
- Take pipette assembly and gently lower syringe into the sample cell. Proceed slowly as the syringe can easily bend, so great care is needed. Ensure the syringe is completely inserted by pressing down on base of locking collar.
- Set the desired running temperature (in this case 20°C), and select a reference power that is slightly greater than the maximum injection heat flow expected (in this case 20μcal/sec).
- Program the desired injection volumes and delays. (In this case, 28 injections were employed. The first injection had a volume of 2 μL with a 60 s delay. All subsequent injections had volumes of 10 μL with 330 s delays.)
6. Subsequent Runs
- In all subsequent runs repeat steps 2-5, while decreasing AAC(6')-Ii and AcCoA concentrations by a factor of 2,4,8,16, and 32.
7. Data Analysis
- Use a fitting procedure which globally fits all of the isotherms to a single set of binding parameters, as described previously10.
8. Representative Results
Representative data are shown in Figure 1. The shapes of the isotherms should vary with concentration. Sharper transitions are expected for higher c-values (i.e. higher protein and ligand concentrations) (Figure 2).
In the case of AAC(6')-Ii, the two-site sequential model gives a better fit than one describing two sets of identical, independent sites with adjustable stoichiometries.
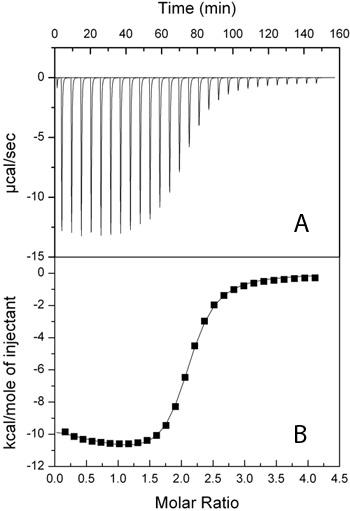
Figure 1. Isotherms produced by titration of AcCoA (3.86 mM) into AAC(6')-Ii (192 μM). A) Raw ITC trace. B) Integrated values used for determining binding parameters (squares) with a 2-site sequential fit (-).

Figure 2. ITC isotherms for AcCoA titrated into AAC(6')-Ii at varying concentrations. The experimental data (open circles) were fit to a 2-sets-of-sites independent model (dashed magenta) and a 2-site sequential model (solid blue). The 2-site sequentional model clearly gives better overall agreement. The concentrations employed were A) 6 μM, 0.25 mM, B) 12 μM, 0.25 mM, C) 24 μM, 0.5 mM, D) 48 μM, 1.0 mM, E) 96 μM, 1.9 mM, and F) 196 μM, 3.86 mM, for AAC(6')-Ii and AcCoA respectively.
Discussion
This analytical portion of variable-c fitting has been previously described in detail10. Here we report practical aspects of collecting variable-c datasets suitable for this approach. It is essential that all protein and ligand samples are drawn from the same stock solutions. Therefore it is important that sufficient stock solution is prepared initially to complete the entire series of experiments. This ensures the ratio of AAC(6')-Ii and AcCoA is constant among all experiments, and reduces random fluctuation...
Disclosures
No conflicts of interest declared.
Acknowledgements
This work was supported by the Canadian Institutes of Health Research (CIHR), National Science and Engineering Research Council (NSERC), and a CIHR training grant scholarship (to L.F.). We thank Prof. Gerard D. Wright (McMaster University, Canada) for the AAC(6)-Ii expression plasmid.
Materials
| Name | Company | Catalog Number | Comments |
| Acetyl c–nzyme A (AcCoA) | Sigma-Aldrich | A2056 | |
| 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) | Fisher Scientific | 7365-45-9 | |
| ethylenediaminetetraacetic acid (EDTA) | Sigma-Aldrich | 431788 | |
| Spectra/Por 2 Dialysis Tubing | Spectrum Labs | 132678 | |
| Sterile Syringe Filter (0.2 μm) | VWR international | 281445-477 | |
| Cellulos Nitrate Membrane Filters (0.45 μm) | Whatman, GE Healthcare | 7184-004 | |
| VP-ITC | MicroCal | VP-ITC | Microcalorimeter used for measurements |
| ThermoVac | MicroCal | USB Thermo Vac | Temperature Controlled Degassing Station |
References
- Cliff, M. J., Ladbury, J. E. A survey of the year 2002 literature on applications of isothermal titration calorimetry. Journal of Molecular Recognition. 16, 383-391 (2003).
- Cliff, M. J., Gutierrez, A., Ladbury, J. E. A survey of the year 2003 literature on applications of isothermal titration calorimetry. Journal of Molecular Recognition. 17, 513-523 (2004).
- Ababou, A., Ladbury, J. E. Survey of the year 2004: literature on applications of isothermal titration calorimetry. Journal of Molecular Recognition. 19, 79-89 (2006).
- Ababou, A., Ladbury, J. E. Survey of the year 2005: literature on applications of isothermal titration calorimetry. Journal of Molecular Recognition. 20, 4-14 (2007).
- Okhrimenko, O. k. s. a. n. a., J, I. A survey of the year 2006 literature on applications of isothermal titration calorimetry. Journal of Molecular Recognition. 21, 1-19 (2008).
- Bjelic, S., Jelesarov, I. A survey of the year 2007 literature on applications of isothermal titration calorimetry. Journal of Molecular Recognition. 21, 289-312 (2008).
- Leavitt, S., Freire, E. Direct measurement of protein binding energetics by isothermal titration calorimetry. Current Opinion in Structural Biology. 11, 560-566 (2001).
- Wiseman, T., Williston, S., Brandts, J. F., Lin, L. -. N. Rapid measurement of binding constants and heats of binding using a new titration calorimeter. Analytical Biochemistry. 179, 131-137 (1989).
- Capaldi, S. The X-Ray Structure of Zebrafish (Danio rerio) Ileal Bile Acid-Binding Protein Reveals the Presence of Binding Sites on the Surface of the Protein Molecule. Journal of Molecular Biology. 385, 99-116 (2009).
- Freiburger, L. A., Auclair, K., Mittermaier, A. K. Elucidating Protein Binding Mechanisms by Variable-c ITC. ChemBioChem. 10, 2871-2873 (2009).
- Wybenga-Groot, L. E., Draker, K. -. a., Wright, G. D., Berghuis, A. M. Crystal structure of an aminoglycoside 6'-N-acetyltransferase: defining the GCN5-related N-acetyltransferase superfamily fold. Structure. 7, 497-507 (1999).
- Draker, K., Northrop, D. B., Wright, G. D. Kinetic Mechanism of the GCN5-Related Chromosomal Aminoglycoside Acetyltransferase AAC(6')-Ii from Enterococcus faecium: Evidence of Dimer Subunit Cooperativity. Biochemistry. 42, 6565-6574 (2003).
- Wright, G. D., Ladak, P. Overexpression and characterization of the chromosomal aminoglycoside 6'-N-acetyltransferase from Enterococcus faecium. Antimicrob. Agents Chemother. 41, 956-960 (1997).
- MicroCal. . ITC Data Analysis in Origin. , (2004).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved