A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Cannulation of the Mouse Submandibular Salivary Gland via the Wharton's Duct
In This Article
Summary
A protocol for the cannulation of the mouse submandibular salivary gland via the Wharton's duct is described. For this experiment, the trypan blue solution is used as a dyer to demonstrate how this technique effectively delivers infusions into the targeted gland, and to suggest the reliability of this new approach as a potential clinical drug/cell therapy for the regeneration of salivary glands.
Abstract
Severe salivary gland hypofunction is frequently found in patients with Sjögren's syndrome and those who receiving therapeutic irradiation in their head and neck regions for cancer treatment. Both groups of patients experience symptoms such as xerostomia (dry mouth), dysphagia (impaired chewing and swallowing), severe dental caries, altered taste, oro-pharyngeal infections (candidiasis), mucositis, pain and discomfort.
One innovative approach of regenerative medicine for the treatment of salivary gland hypo-function is speculated in RS Redman, E Mezey et al. 2009: stem cells can be directly deposited by cannulation into the gland as a potent method in reviving the functions of the impaired organ. Presumably, the migrated foreign stem cells will differentiate into glandular cells to function as part of the host salivary gland. Also, this cannulation technique is an expedient and effective delivery method for clinical gene transfer application.
Here we illustrate the steps involved in performing the cannulation procedure on the mouse submandibular salivary gland via the Wharton's duct (Fig 1). C3H mice (Charles River, Montreal, QC, Canada) are used for this experiment, which have been kept under clean conventional conditions at the McGill University animal resource center. All experiments have been approved by the University Animal Care Committee and were in accordance with the guidelines of the Canadian Council on Animal Care.
For this experiment, a trypan blue solution is infused into the gland through the opening of the Wharton's duct using a insulin syringe with a 29-gauge needle encased inside a polyethylene tube. Subsequently, the mouse is dissected to show that the infusions migrated into the gland successfully.
Protocol
1. Procedural Description
- Subcutaneously inject 1 μl / g body weight of 0.5 mg / ml atropine sulfate monohydrate (Cat# 11330, Fluka, USA) into each mouse to prevent the salivary secretions from disrupting the movement of the infusions.
- Anesthetize each mouse with 1 μl / g body weight of a 100 mg / ml ketamine and 20 mg / ml xylazine (Phoenix Scientific, AR, USA) solution injected intra-peritoneal (I.P.)
- Place the mouse on a custom-made plastic platform in the ventral position: the maxillary incisors are locked on a metal wire, and the mandibular incisors are hooked on an elastic string in order to hold the mouth open.
- Use an Insulin syringe with a 29-gauge needle (Cat# 600145, Tyco Healthcare, MA, USA) placed inside a 0.58 mm diameter polyethylene tube (Cat# 427410, Becton Dickinson, MD, USA; Fig 2). The polyethylene tube is inserted 5mm over the tip of a 29-gauge needle. The needle is used as a rigid guide to move the polyethylene tube into the Wharton's duct. The polyethylene tube is inserted 3-5 mm inside the duct. Note that only the polyethylene tube enters the salivary gland duct while the needle remains outside the duct. Inject 50ul of filtered 0.4% trypan blue stain solution (Cat# 15250, Gibco, USA) into the submandibular gland via the Wharton's duct, which is located right beneath the tongue. The infusion is delivered steadily with positive pressure on the syringe piston; the finger remains on the piston even after all of the infusion has been delivered to the gland, in order to prevent a backflow of the infusion intra-orally. The trypan blue solution is used in the video as a dye to demonstrate the technique.
- fter the injection of the trypan blue solution, the mouse was euthanized prior to dissection. The salivary gland is surgically exposed in order to determine how much of the infusions have actually remained in the gland.
2. Representative Results:
After the infusions were injected, the mouse was dissected to surgically expose the post-cannulated salivary gland. As shown on Image-2, the dark coloration of the Wharton's duct provides evidence that the trypan blue solution was infused successfully.
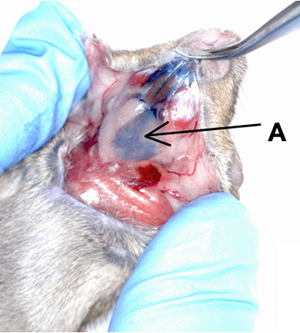
Figure 1. Cannulation of mouse submandibular gland.
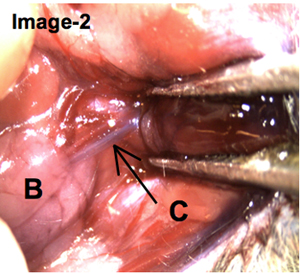
Figure 2. Polyethylene tube is encased around the tip of a 29-gauge needle. The syringe needle is used as a rigid guide to move the polyethylene tube into the Wharton's duct. The polyethylene tube is inserted (cannulated) 3-5 mm inside the duct. Note that only the polyethylene tube enters the salivary gland duct while the needle remains outside the duct.
Discussion
A difficulty using this cannulation method is to prevent the backflow of the injected solution. Even if the trypan blue solution has temporarily migrated into the salivary gland successfully, the saliva secretion might interfere with the infusions and cause backflow of the trypan blue solution out of the gland. As a precaution, in addition to using atropine to inhibit secretion of saliva, the polyethylene cannula should remain in the Wharton's duct and the piston of the syringe held for 60 seconds after the infusions hav...
Disclosures
All experiments have been approved by the University Animal Care Committee and were in accordance with the guidelines of the Canadian Council on Animal Care.
Acknowledgements
The authors are grateful to Dr. Ana Cotrim for technical consultation and to Dr. Monzur Murshed for equipment. This work was in part funded by the Canadian Institutes for Health Research.
Materials
| Name | Company | Catalog Number | Comments | |
| Atropine | Drug | Fluka | 11330 | To inhibit saliva secretion |
| Ketamine | Drug | Phoenix Scientific | Sedation | |
| Xylazine | Drug | Phoenix Scientific | ||
| Polyethylene tubing (0.58 mm diameter) | Tube | Intramedic | 427410 | To insert over tip of needle to avoid tissue injury during insertion into Wharton’s duct. |
| Ultra Comfort Insulin syringe (with a 29G needle) | Syringe | Tyco Healthcare, Covidien | 600145 | |
| 4% trypan blue | Stain | GIBCO, by Life Technologies | 15250 |
References
- Fox, P. C. Acquired salivary dysfunction. Drugs and radiation. Ann N Y Acad Sci. 842, 132-137 (1998).
- Bhide, S. A., Miah, A. B., Harrington, K. J., Newbold, K. L., Nutting, C. M. Radiation-induced xerostomia: pathophysiology, prevention and treatment. Clin Oncol (R Coll Radiol). 21, 737-744 (2009).
- Redman, R. S., Ball, W. D., Mezey, E., Key, S. Dispersed donor salivary gland cells are widely distributed in the recipient gland when infused up the ductal tree. Biotech Histochem. 84, 253-260 (2009).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved