A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
The Use of Thermal Infra-Red Imaging to Detect Delayed Onset Muscle Soreness
In This Article
Summary
The purpose of this investigation was to assess whether using an infra-red thermal camera is a valid tool for detecting and quantifying the muscle soreness after exercising.
Abstract
Delayed onset muscle soreness (DOMS), also known as exercise induced muscle damage (EIMD), is commonly experienced in individuals who have been physically inactive for prolonged periods of time, and begin with an unexpected bout of exercise1-4, but can also occur in athletes who exercise beyond their normal limits of training5. The symptoms associated with this painful phenomenon can range from slight muscle tenderness, to severe debilitating pain1,3,5. The intensity of these symptoms and the related discomfort increases within the first 24 hours following the termination of the exercise, and peaks between 24 to 72 hours post exercise1,3. For this reason, DOMS is one of the most common recurrent forms of sports injury that can affect an individual’s performance, and become intimidating for many1,4.
For the last 3 decades, the DOMS phenomenon has gained a considerable amount of interest amongst researchers and specialists in exercise physiology, sports, and rehabilitation fields6. There has been a variety of published studies investigating this painful occurrence in regards to its underlying mechanisms, treatment interventions, and preventive strategies1-5,7-12. However, it is evident from the literature that DOMS is not an easy pathology to quantify, as there is a wide amount of variability between the measurement tools and methods used to quantify this condition6. It is obvious that no agreement has been made on one best evaluation measure for DOMS, which makes it difficult to verify whether a specific intervention really helps in decreasing the symptoms associated with this type of soreness or not. Thus, DOMS can be seen as somewhat ambiguous, because many studies depend on measuring soreness using a visual analog scale (VAS)10,13-15, which is a subjective rather than an objective measure. Even though needle biopsies of the muscle, and blood levels of myofibre proteins might be considered a gold standard to some6, large variations in some of these blood proteins have been documented 6,16, in addition to the high risks sometimes associated with invasive techniques.
Therefore, in the current investigation, we tested a thermal infra-red (IR) imaging technique of the skin above the exercised muscle to detect the associated muscle soreness. Infra-red thermography has been used, and found to be successful in detecting different types of diseases and infections since the 1950’s17. But surprisingly, near to nothing has been done on DOMS and changes in skin temperature. The main purpose of this investigation was to examine changes in DOMS using this safe and non-invasive technique.
Protocol
1. The Exercise
- The muscle of interest for this experiment was the elbow flexors (Biceps Brachii).
- Muscle strength was measured for each participant to be able to give each individual an appropriate resistance. This was determined by testing each participant for their resistance maximum (RM).
- For testing the RM, we used a strain gauge device interfaced with a computer through a BioPac (DA-100C) bioelectric amplifier module (BioPac Systems, Goleta, CA) to measure muscle strength. The module was connected to an MP-100 analog to digital converter sampling at a frequency of 1,000 hertz per second, and at a resolution of 24 bits (Fig. 10).
- The strain gauge device was fixed to a bench at a 45° angle. The subjects were instructed to sit behind the device and rest their elbows on the padded area, so that the exertion force is through their wrists. This was the best way to insure that the subject will not recruit any muscle other than the biceps (Fig. 11).
- Strength was determined on 3 occasions with each contraction being 3 seconds in duration with approximately 45 seconds separating the contractions. The average of the 3 measurements was the RM.
- After determining the RM for the biceps muscle of each participant, the intended session of exercise was carried out with 35% of their RM.
- All the subjects underwent the same exercise using appropriate weighted dumbbells to induce the muscle soreness (DOMS). This was carried out by doing 4 sets of 25 repetitions of biceps concentration curls while seated on a chair, and with the elbows supported on their thighs (Fig. 12).
- Each subject was given a 90 second resting period between each set. Subjects either did the full set of 25 repetitions, or were instructed to stop if they failed to steadily control the weight during the exercise.
2. Infra-Red Camera Preparation & Setup
- The room where the infra-red imaging takes place was set at a constant temperature to minimize any external bias from differences in room temperature, which could lead to false thermal readings. For the purpose of this experiment we had a temperature controlled room which was maintained at approximately 23°C (+/- 0.5°C).
- The camera was set at a distance of 1 meter away, and at a perpendicular angle to the skin being measured (Fig. 9a)*.
- After the required distance was set up, the subjects were advised to stand still until the image has been taken. This shouldn't take more than a couple of seconds, but it is very critical to minimize movement to insure the accuracy of the taken image.
- It is preferable that the room has darker colored paint, rather than lighter colors, to minimize any infra-red interference.
- Lighting is also critical when dealing with infra-red images, because light source that emit infra-red waves like fluorescent or tungsten lighting could give false high readings. The best lighting option would be a room equipped with uniform LED lights, as LED lights hardly produce any infra-red interference (Fig. 9b)*.
* A series of tests were done at our labs using the FLIR 660 IR Camera (Fig. 8), where we compared images of the skin at different angles (0 (perpendicular), 15, 30, 45, and 60 degrees), and at different distances (1, 2, and 5 meters) from the skin, to accurately detect the temperature of the skin. All images were compared to calibrated thermocouples, and the best correlation between the images and the thermocouple readings was at a perpendicular angle and at a distance of 1 meter away from the skin (r = 0.93). The different angles and distances caused a pixilation loss, and decreased the overall correlation between the images and the thermocouple readings.
3. Image Acquirement
- For the purpose of this experiment, the image of the exercised muscle was taken before the exercise, and at 24, and 48 hours post exercise.
- Body heat from sources other than the target could disrupt the thermal image and give false readings. For this reason, no one should be standing beside or behind the intended target.
- In this investigation, pictures of both the exercised and non-exercised arm were taken for comparison purposes. We exercised one of the arms, as was mentioned previously, and the other arm was used as a control (Fig. 4 and 5).
- Image numbers from the IR camera were recorded immediately on a separate spreadsheet, as it could be difficult to identify which image belongs to whom.
4. Image Processing & Analyses
- The acquired IR images were processed using the "ThermoVision ExaminIR" software Version: 1.10.2.
- After selecting the required image for analysis, four regions of interest were identified on the acquired image of the arm using statistical boxes on the software interface (Fig.6).
- When the required regions across the arm have been located, the software shows the Means and Standard Deviations of the temperatures for each of the selected regions. We can then either cross compare each region individually or obtain an average temperature of the whole arm (Fig. 7).
5. Visual Analog Scale & Blood Analysis
- A visual analog scale (VAS) was used to assess subjective soreness of the arm. The scale had a 10 cm (100mm) long line marked "no pain" at one end, and "extremely sore" at the opposite end. Each participant was directed to make a mark along the 10 cm line to indicate their response to soreness.
- The VAS's were administered to the subjects before the exercise, 24 hours after the exercise, and at 48 hours.
- Peripheral blood was collected from the subjects to measure myoglobin concentration levels in the blood.
- The blood was drawn from the subjects antecubital vein before the exercise, 30 minutes after the exercise was over, and at 48 hours.
- The blood was centrifuged at 4000 rpm for 10 min to separate the serum from the cells. The samples were then stored at -80°C until the analyses of myoglobin was done.
- Myoglobin was measured using a TOSOH "AIA-360" Automated enzyme Immunoassay Analyzer (TOSOH Corp., Tokyo, Japan). The myoglobin Assay kits (Myo 025297, ST AIA-PACK Myoglobin) were used according to the manufactures instructions.
6. Representative Results
The results of IR thermal images taken during this investigation are clearly represented in figure 1. Images taken at the 3 time periods (pre-exercise, 24 hours post-exercise, and 48 hours post-exercise) for the exercised arms of the 41 subjects, showed a noticeable increase in temperature on day 2 (24 hours post-exercise) when compared to pre-exercise temperatures, and temperatures taken at 48 hours. As shown in figure 1, the average skin temperature was 32.80 +/- 1.03 °C for day 1 (pre-exercise), and 33.96 +/- 1.46 °C for day 2 (24 hours post-exercise), and 32.82 +/- 1.29 for day 3 (48 hours post-exercise). This difference in skin temperature from day 1 to day 2 was significant (ANOVA p < 0.01).
However, for the un-exercised arm, changes amongst the 3 time periods were not evident. Figure 1 shows that the average skin temperature was 33.08 +/- 0.83 °C for day 1 (pre-exercise), and 32.79 +/- 1.42 °C for day 2 (24 hours post-exercise), and 33.17 +/- 0.95 for day 3 (48 hours post-exercise). This difference in skin temperature over the 3 days was not significant (ANOVA p = 0.38).
The results of the pain readings from the VAS are shown in figure 2. As seen in figure 2, the reported pain had a dramatic increase on days 2 and 3. Pain levels of the exercised muscle increased from 3.6 +/- 6.1 on day 1, to 36.3 +/- 22.8 on day 2, and 37.5 +/- 25.3 on day 3. This increase from day 1 was significant (ANOVA p < 0.01).
The results of the myoglobin concentration levels are shown in figure 3. As seen in this figure, there was hardly any change between the 2 myoglobin concentrations on day 1 (pre, & 30 minutes post exercise). But on day 3, the increase in myoglobin was very large. This increase on day 3 was approximately 147 nanograms per milliliter (ng/mL) of blood when compared to the first 2 concentrations on day 1. Myoglobin concentrations were 30.12 +/- 7.66 ng/mL at baseline, 31.66 +/- 11.89 ng/mL 30 minutes post exercise, and 178.96 +/- 249.51 ng/mL on day 3. This increase on day 3 was highly significant (ANOVA p < 0.01).
A correlation analysis was done between the skin temperatures obtained from the IR images, and the VAS soreness levels. It was found that there was a considerable correlation between the VAS readings on day 2, and the skin temperature measurement on day 2. This correlation was significant (r = 0.312, p < 0.05). However, there was no evident correlation between the VAS readings and the skin temperatures on day 3. This correlation was insignificant (r = 0.047, p = 0.77).
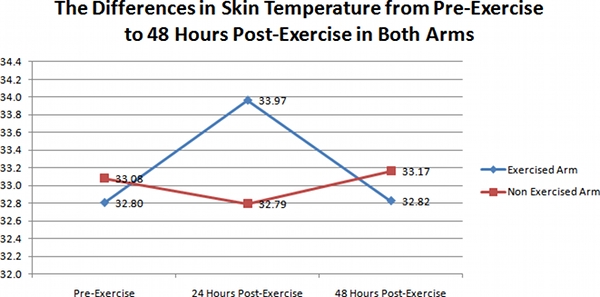
Figure 1. A representative graph of the differences in skin temperature in the exercised arms (Diamonds), and un-exercised arms (Squares) of the 41 subjects over the 3 day time period.
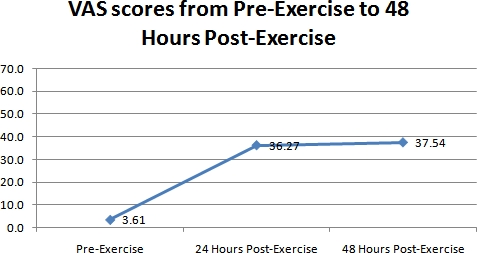
Figure 2. A representative graph of the differences in perceived muscle soreness measured with the VAS over the 3 day time period for all the 41 subjects.
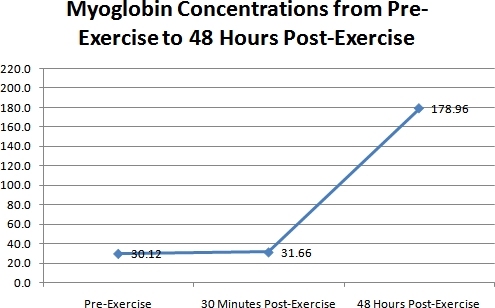
Figure 3. A representative graph of the differences in myoglobin concentrations for all the 41 subjects over the 3 time periods.
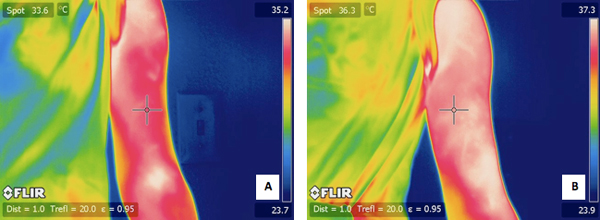
Figure 4. A) a typical IR image of a subject's exercised arm before the exercise. B) an IR image of the same subjects arm 24 hours after the exercise.
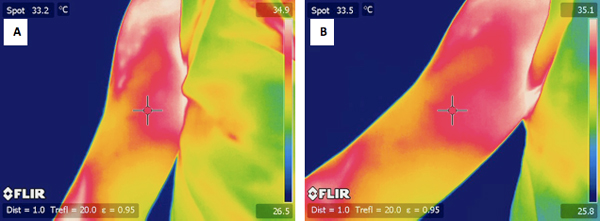
Figure 5. A) a typical IR image of a subject's un-exercised arm before the exercise. B) an IR image of the same subjects arm 24 hours after the exercise.
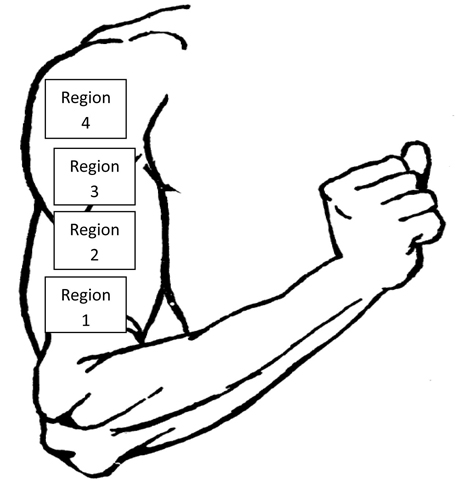
Figure 6. An illustration of the 4 regions of interest for analyzing the thermal image of the arm.
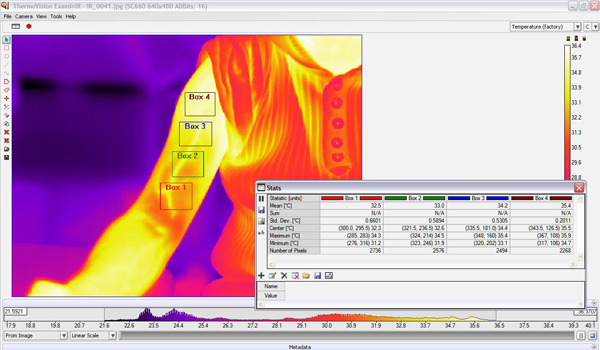
Figure 7. Software interface for the "ThermoVision ExaminIR" showing the 4 boxes of interest on an IR image of an exercised arm. Also shown are the statistical interpretations for each box.

Figure 8. The IR thermal camera used for this investigation (FLIR 660).
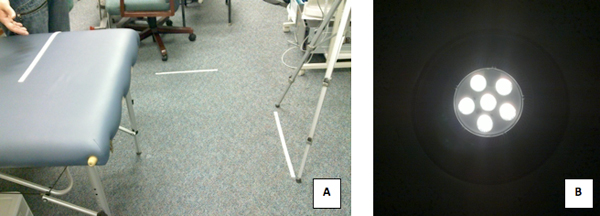
Figure 9. A) The Setup of the IR Camera 1 meter away from the subjects arm. B) The LED lights used in the lab where the images were taken.

Figure 10. A) The BioPac Modules used for measuring the muscle strength. B) The strain gauge device fixed to a 45° angled bench and hooked to the BioPac system.
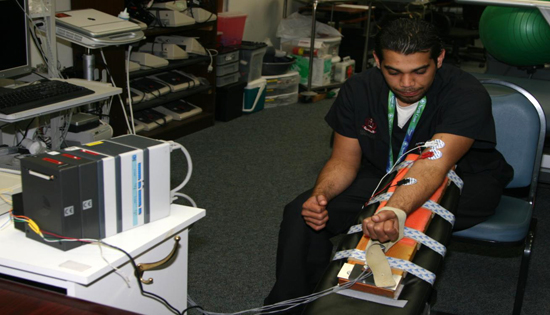
Figure 11. A typical subject exerting force on the strain gauge device.
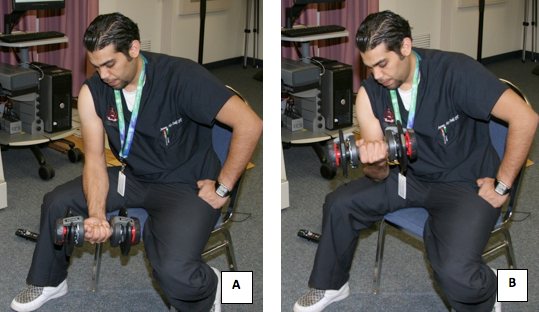
Figure 12. A subject undergoing the exercise protocol for inducing the muscle soreness.
Discussion
The primary purpose of this investigation was to assess the usefulness of thermal IR imaging in detecting and measuring muscle soreness after strenuous exercise, and our results suggest that IR imaging could be a valid technique for detecting DOMS, especially within the first 24 hours of exercising. This is not surprising, as Pennes18 provided a very detailed model of heat flow from muscle to skin in limbs. This model predicts that heat in deeper tissues such as muscles can be dissipated into blood and into th...
Disclosures
No conflicts of interests declared.
Acknowledgements
We wish to acknowledge a contract (WS1763368) from Pfizer Pharmaceuticals for support in this work. We would also like to thank the Saudi Arabian Ministry of Higher Education (MOHE) for their support.
Materials
| Name | Company | Catalog Number | Comments |
| Infra-Red Thermal Camera | FLIR Systems Inc. | FLIR SC660 | |
| Thermal Infra-Red Analysis Software | Thermo Fisher Scientific, Inc. | Software Version 1.10.2 | |
| Bi–lectric Amplifier Module | Biopac Systems, Inc. | DA100C | The DA100C provides variable gain settings, and adjustable voltage references. |
| Analog to Digital Converter Module | Biopac Systems, Inc. | MP100 | |
| Automated enzyme Immunoassay Analyzer | Tosoh Corp. | AIA -360 | This device was used to analyze the blood samples, and obtain the myoglobin readings. |
References
- Cheung, K., Hume, P. A., Maxwell, L. Delayed Onset Muscle Soreness: Treatment strategies and Performance Factors. Sports. Med. 33, 145-164 (2003).
- MacIntyre, D. L., Reid, W. D., McKenzie, D. C. Delayed Muscle Soreness: The Inflammatory Response to Muscle Injury and its Clinical Implications. Sports. Med. 20, 24-40 (1995).
- Armstrong, R. B. Mechanisms of exercise-induced delayed onset muscular soreness: a brief review. Medicine and Science in Sports and Exercise. 16, 529-538 (1984).
- Howatson, G., Someren, K. A. V. The Prevention and Treatment of Exercise-Induced Muscle Damage. Sports. Med. 38, 483-503 (2008).
- Petrofsky, J. Comparison of Different Heat Modalities for Treating Delayed-Onset Muscle Soreness in People with Diabetes. Diabetes Technology & Therapeutics. 13, 645-655 (2011).
- Warren, G. L., Lowe, D. A., Armstrong, R. B. Measurement Tools Used in the Study of Eccentric Contraction-Induced Injury. Sports. Med. 27, 43-59 (1999).
- Hilbert, J. E., Sforzo, G. A., Swensen, T. The Effects of Massage on Delayed Onset Muscle Soreness. Br. J. Sports. Med. 37, 72-75 (2003).
- Symons, T. B., Clasey, J. L., Gater, D. R., Yates, J. W. Effects of Deap Heat as a Preventative Mechanism on Delayed Onset Muscle Soreness. Journal of Strength and Conditioning Research. 18, 155-161 (2004).
- Vaile, J. M., Gill, N. D., Blazevich, A. J. The Effect of Contrast Water Therapy on Symptoms of Delayed Onset Muscle Soreness. Journal of Strength and Conditioning Research. 21, 697-702 (2007).
- Stone, M. B., Merrick, M. A., Ingersoll, C. D., Edwards, J. E. Preliminary Comparison of Bromelain and Ibuprofen for Delayed Onset Muscle Soreness Management. Clinical Journal of Sports Medicine. 12, 373-378 (2002).
- Barlas, P. Managing Delayed-Onset Muscle Soreness: Lack of Effect of Selected Oral Systemic Analgesics. Arch. Phys. Med. Rehabil. 81, 966-972 (2000).
- Jackman, S. R., Witard, O. C., Jeukendrup, A. E., Tipton, K. D. Branched-Chain Amino Acid Ingestion Can Ameliorate Soreness from Eccentric Exercise. Medicine & Science in Sports & Exercise. 42, 962-970 (2010).
- Law, F. r. e. y., A, L. Massage Reduces Pain Perception and Hyperalgesia in Experimental Muscle Pain: A Randomized, Controlled Trial. The Journal of Pain. 9, 714-721 (2008).
- Vaile, J., Halson, S., Gill, N., Dawson, B. Effect of hydrotherapy on the signs and symptoms of delayed onset muscle soreness. European Journal of Applied Physiology. 102, 447-455 (2007).
- Vinck, E., Cagnie, B., Coorevits, P., Vanderstraeten, G., Cambier, D. Pain reduction by infrared light-emitting diode irradiation: a pilot study on experimentally induced delayed-onset muscle soreness in humans. Lasers in Medical Science. 21, 11-18 (2006).
- Clarkson, P. M., Ebbeling, C. Investigation of Serum Creatine Kinase Variability after Muscle-Damaging Exercise. Clin. Sci. 75, 257-261 (1988).
- Jiang, L. J. A perspective on medical infrared imaging. Journal of Medical Engineering & Technology. 29, 257-267 (2005).
- Pennes, H. H. Analysis of Tissue and Arterial Blood Temperatures in the Resting Human Forearm. J. Appl. Physiol. 1, 93-122 (1948).
- Ivanitsky, G. R., Khizhnyak, E. P., Deev, A. A., Khizhnyak, L. N. Thermal imaging in medicine: A comparative study of infrared systems operating in wavelength ranges of 3–5 and 8-12 μm as applied to diagnosis. Doklady Biochemistry and Biophysics. 407, 59-63 (2006).
- Herman, C., Cetingul, M. P. Quantitative Visualization and Detection of Skin Cancer Using Dynamic Thermal Imaging. J. Vis. Exp. (51), e2679-e2679 (2011).
- Wang, J. Evaluation of the diagnostic performance of infrared imaging of the breast: a preliminary study. BioMedical Engineering OnLine. 9, 3-3 (2010).
- Murray, A. K. Noninvasive imaging techniques in the assessment of scleroderma spectrum disorders. Arthritis & Rheumatism. 61, 1103-1111 (2009).
- Zaproudina, N., Ming, Z., Hanninen, O. O. P. Plantar Infrared Thermography Measurements and Low Back Pain Intensity. Journal of Manipulative and Physiological Therapeutics. 29, 219-223 (2006).
- Kim, Y. -. C., Bahk, J. -. H., Lee, S. -. C., Lee, Y. -. W. Infrared Thermographic Imaging in the Assessment of Successful Block on Lumbar Sympathetic Ganglion. Yonsei Medical Journal. 44, 119-124 (2003).
- Brancaccio, P., Lippi, G., Maffulli, N. Biochemical markers of muscular damage. Clinical Chemistry and Laboratory Medicine. 48, 757-767 (2010).
- Neubauer, O., König, D., Wagner, K. -. H. Recovery after an Ironman triathlon: sustained inflammatory responses and muscular stress. European Journal of Applied Physiology. 104, 417-426 (2008).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved