A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Submillisecond Conformational Changes in Proteins Resolved by Photothermal Beam Deflection
In This Article
Summary
Here we report an application of the photothermal beam deflection technique in combination with a caged calcium compound, DM-nitrophen, to monitor microsecond and millisecond dynamics and energetics of structural changes associated with the calcium association to a neuronal calcium sensor, Downstream Regulatory Element Antagonist Modulator.
Abstract
Photothermal beam deflection together with photo-acoustic calorimetry and thermal grating belongs to the family of photothermal methods that monitor the time-profile volume and enthalpy changes of light induced conformational changes in proteins on microsecond to millisecond time-scales that are not accessible using traditional stop-flow instruments. In addition, since overall changes in volume and/or enthalpy are probed, these techniques can be applied to proteins and other biomacromolecules that lack a fluorophore and or a chromophore label. To monitor dynamics and energetics of structural changes associated with Ca2+ binding to calcium transducers, such neuronal calcium sensors, a caged calcium compound, DM-nitrophen, is employed to photo-trigger a fast (τ < 20 μsec) increase in free calcium concentration and the associated volume and enthalpy changes are probed using photothermal beam deflection technique.
Introduction
Photo-thermal methods such as photoacoustic calorimetry, photothermal beam deflection (PDB), and transient grating coupled with nanosecond laser excitation represent a powerful alternative to transient optical spectroscopies for time-resolved studies of short-lived intermediates1,2. In contrast to optical techniques, such as transient absorption and IR spectroscopy, that monitor the time profile of absorption changes in the chromophore surrounding; photothermal techniques detect the time-dependence of heat/volume changes and therefore are valuable tools for investigating time profiles of optically "silent" processes. So far, photoacoustic calorimetry and transient grating has been successfully applied to study conformational dynamics of photo-induced processes including diatomic ligand migration in globins3,4, ligand interactions with oxygen sensor protein FixL5, electron and proton transport in heme-copper oxidases6 and photosystem II as well as photo-isomerization in rhodopsin7 and conformational dynamics in cryptochrome8.
To expand the application of photothermal techniques to biological systems that are lacking an internal chromophore and/or fluorophore, the PBD technique was combined with the use of caged compound to photo-trigger an increase in ligand/substrate concentration within few microseconds or faster, depending on the caged compound. This approach allows monitoring of dynamics and energetics of structural changes associated with the ligand/substrate binding to proteins that are lacking an internal fluorophore or chromophore and on time-scale that are not accessible by commercial stop-flow instruments. Here an application of PBD to monitor the thermodynamics of the cage compound, Ca2+DM-nitrophen, photo-cleavage as well as the kinetics for Ca2+ association to the C-terminal domain of the neuronal calcium sensor Downstream Regulatory Element Antagonist Modulator (DREAM) is presented. The Ca2+ is photo-released from Ca2+DM-nitrophen within 10 μsec and rebinds to an unphotolysed cage with a time constant of ~300 μsec. On the other hand, in the presence of apoDREAM an additional kinetic occurring on the millisecond time-scale is observed and reflects the ligand binding to the protein. The application of PBD to probe conformational transitions in biological systems has been somehow limited due the instrumental difficulties; e.g. arduous alignment of the probe and pump beam to achieve a strong and reproducible PBD signal. However, a meticulous design of an instrumentation set-up, a precise control of the temperature, and a careful alignment of the probe and pump beam provide a consistent and robust PBD signal that allows monitoring of time-resolved volume and enthalpy changes on a broad time-scale from 10 µsec to approximately 200 msec. In addition, modifications of the experimental procedure to assure the detection of sample and reference traces under identical temperature, buffer composition, optical cell orientation, laser power, etc. significantly reduces the experimental error in measured reaction volumes and enthalpies.
Access restricted. Please log in or start a trial to view this content.
Protocol
1. Sample Preparations
- Carry out the sample preparation and all sample manipulations in a dark room to prevent an unwanted uncaging.
- Solubilize DM-nitrophen ((1-(2-nitro-4,5-dimethoxyphenyl)-N,N,N',N'-tetrakis [(oxy-carbonyl) methyl]-1,2-ethanediamine) in 50 mM HEPES buffer, 100 mM KCl, pH 7.0 to a final concentration of 400 µM (ε350nm = 4330 M-1cm-1 9).
- Add CaCl2 from the 0.1 M stock solution to achieve a desirable ratio of [Ca2+]:[DM-nitrophen]. For proteins with Kd for Ca2+ association larger than 10 µM, the ratio of [Ca2+]:[DM-nitrophen] of 1:1 is preferable to prevent binding of photo-released Ca2+ to uncaged DM-nitrophen. Indeed, considering the Kd value for DM-nitrophen to be 10 nM and the total concentration of DM-nitrophen and Ca2+ to be 400 mM, ~90% of a calcium binding protein with Kd = 10 μM will be in the apoform. On the other hand, for study of Ca2+ binding to proteins with Kd < 10 µM, it's preferable to decrease the [Ca2+]:[DM-nitrophen] ratio to 0.95 to prevent Ca2+ complexation with the apo-protein prior the cage photo-dissociation.
- Solubilize the reference compound, K3[Fe(III)(CN)6] or Na2CrO4, in the same buffer as for the sample.
2. Setting up the Experiment
- The basic experimental configuration is shown in Figure 2.
- Use a pin hole (P2) to adjust the diameter of the probe beam (632 nm output of He-Ne laser, ~5 mW laser power) to 1 mm and propagate the probe beam through the center of a cell placed in a temperature controlled cell holder using a M1 mirror.
- Use a mirror (M2) behind the sample to center the probe beam on a center of position sensitive detector.
- Focus the probe beam on the center of the detector in such way that the difference in the voltage between the top two diodes and bottom two diodes as well as the difference in the voltage between the two diodes on the left and right side of the detector is zero.
- Subsequently, shape a diameter of the pump beam, a 355 nm output of Q-switched Nd:YAG laser, FWHM 5 nsec) using a 3 mm pinhole (P1) placed between two 355 nm laser mirrors.
- Copropagate the pump beam through the center of the cuvette as demonstrated in Figure 2. It is important that both laser beams are propagated through the center of the optical cell in nearly colinear manner to obtain a measurable deflection angle and thus high amplitude of PBD signal. Under experimental conditions, the angle of the intersection of the probe and pump beams is less than 15°.
- Use a reference compound to align the probe and pump beam to achieve a satisfactory PBD signal, i.e. a good S/N ratio and stable PBD amplitude on longer time-scales (~100 msec).
- Adjust the position of the pump beam with respect to the probe beam by an incremental adjustment of 355 nm laser mirrors.
- Measure the amplitude of the PBD reference signal as a difference in between two top and bottom photodiodes on the position sensitive detector. The PBD signal should exhibit a rapid increase in the amplitude on a fast time-scale (<10 µsec) and remain stable on 100 msec timescale as demonstrated in Figure 3. The shot to shot variability of the PDB amplitude is within 5% of the signal amplitude and the signal reproducibility is mainly affected by vibrations.
- Check the linearity in the PBD signal amplitude with respect to the released heat energy by measuring the linear dependence of the PBD signal on the excitation laser power and on the number of Einsteins absorbed, Ea = (1-10-A), where A corresponds to the reference absorbance at the excitation wavelength.
- Keep the laser power below approximately 1,000 µJ and absorbance of the sample/reference compound at the excitation wavelength less than 0.5 to prevent multiphoton absorption and decrease of the pump beam power, respectively, and ensure linearity of the PBD signal.
3. PBD Measurements
- Start with the measurement of the PBD traces for the reference. Place the solution of the reference compound in a 1.0 cm x 1.0 cm or 1.0 cm x 0.5 cm quartz cell and position the cell in the temperature controlled holder. Both path lengths provide comparable PBD amplitude.
- Detect the reference PBD signal as a function of temperature in the temperature range from 16-35 °C with the temperature increment of 3 °C.
- Upon each temperature change, check the position of the probe beam on the position sensitive detector and readjust the position to the center of the detector if necessary.
- Check the linearity of the PBD signal as a function of the [(dn/dt)/Cpρ] term according to Equation 2.
- Place the sample solution in the same optical cell as for the reference compound keeping the same orientation of the optical cell as for the reference measurement.
- Detect the sample PBD traces in the same temperature range as for the reference and check the linearity of the sample PBD amplitude with respect to the [(dn/dt)/Cpρ] term.
4. Data Analysis
The magnitude of the deflection is directly proportional to the volume change due to the sample heating (ΔVth) and nonthermal volume change (ΔVnonth) according to Equation 1: 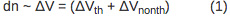
The amplitude of the sample (AS) and reference (Ar) PBD signal can be described using Equations 2 and 3, respectively.
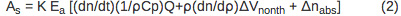
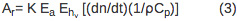
PBD signal is directly proportional to the instrument response parameter (K) and the number of Einsteins absorbed (Ea). The first term in Equation 2, (dn/dt)(1/ρCp)Q, corresponds to the signal change due to the heat released to the solvent. The dn/dt term represents the temperature-dependent change in the index of refraction, ρ is the density of the solvent, Cp is the heat capacity. All parameters are known for the distilled water and can be determined for a buffer solution by comparing a PBD signal for the reference compound in distilled water and in an appropriate buffer. Q is the amount of heat returned to the solvent. The ρ (dn/d ρ ) term is a unit-less constant that is temperature-independent in the temperature range from 10-40 °C 10. Δnabs term corresponds to the change in the index of refraction due to the presence of absorbing species in the solution and it's negligible if the wavelength of the probe beam is shifted relative to the absorption spectra of any species in the solution. The signal arising from the reference compound (Ar) is expressed by Equation 3 where Ehν is the photon energy at the excitation wavelength, Ehν = 80.5 kcal/mol for 355 nm excitation.
- Take the amplitude of the reference PBD signal as the difference between the pretrigger and post trigger PBD signal as demonstrated in Figure 3. In a similar way, determine the amplitude of the fast (Asfast) and slow phase (Asslow) of the sample PBD signal.
- To eliminate the instrument response parameter, K, scale the amplitude of the sample PBD signal by the amplitude of the PBD signal for the reference. The ratio of the sample signal to the reference signal gives Equation 4 and can be written as:

- Using this equation, determine the heat released to the solution (Q) and nonthermal volume change (ΔVnonth) associated with a photo-initiated reaction from the slope and intercept, respectively, of a plot of [(As/Ar) Ehν] term versus temperature dependent term [(dn/dt)(1/ρCp)].
- To determine the reaction volume and enthalpy change for the fast and the slow process, scale the observed volume and enthalpy change to the appropriate quantum yield according to Equations 5-7.

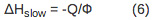
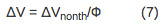
For a multistep process with kinetics occurring on the time-scale between 10 µsec~200 msec, the volume and enthalpy changes associated with the individual steps of the reaction can be determined. The amplitudes and lifetimes for the individual steps are analyzed by fitting the data to the function F(t) that describes the time profile of the volume and enthalpy changes.
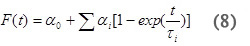
where α0 corresponds to the Asfast and αi corresponds to Asslow for each individual process and τ i are the lifetime of the individual reaction steps. From the temperature-dependence of the rate constant for individual processes (ki = 1/τi) the activation enthalpy and entropy parameters can be readily determined using Eyring plots.
Access restricted. Please log in or start a trial to view this content.
Results
A representative example of PBD traces for Ca2+ photo-release from Ca2+DM-nitrophen is shown in Figure 3. The fast phase corresponds to the photo-cleavage of Ca2+DM-nitrophen and Ca2+ liberation, whereas the slow phase reflects Ca2+ binding to the nonphotolysed cage. The plot of the sample PBD amplitude for the fast and slow phase scaled to the amplitude of the reference compound as a function of the temperature depended factor [Cpρ/(...
Access restricted. Please log in or start a trial to view this content.
Discussion
The physical principle behind photothermal methods is that a photo-excited molecule dissipates excess energy via vibrational relaxation to the ground state, resulting in thermal heating of the surrounding solvent1,12. For solvents such as water, this produces a rapid volume expansion (ΔVth). Excited-state molecules may also undergo photochemical processes that result in nonthermal volume changes (ΔVnonth) due to bond cleavage/formation and/or changes in molecular structure that ...
Access restricted. Please log in or start a trial to view this content.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by National Science Foundation (MCB 1021831, JM) and J. & E. Biomedical Research Program (Florida Department of Health, JM).
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| 1-(4,5-Dimethoxy-2-Nitrophenyl)-1,2-Diaminoethane-N,N,N',N'-Tetraacetic Acid | Life Technologies | D-6814 | DM-nitrophen, cage calcium compound, keep stock solutions in dark to prevent photodissociation |
| 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid, N-(2-Hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid) | Sigma Adrich | 0909C | HEPES buffer |
| Potassium ferricyanide (III) | Sigma Aldrich | 702587 | reference compound for PBD measurements |
| Sodium chromate | Sigma Aldrich | 307831 | reference compound for PBD measurements |
| He-Ne Laser Diode 5 mW 635 nm | Edmund Optics | 54-179 | use as a probe beam for PBD measurements |
| Oscilloscope, | LeCroy | Wave Surfer 42Xs | 400 MHz bandwith |
| Nd:YAG laser | Continuum | ML II | pump beam for PBD measurements |
| M355; Nd:YAG laser mirror | Edmund Optics | 47-324 | laser mirror for 355 nm laser line |
| M1 and M2; Laser diode mirror | Edmund Optics | 43-532 | visilbe laser flat mirror, wavelength range 300-700 nm |
| P1 and P2; Iris Diaphragm | Edmund Optics | 62-649 | pin hole to shape the probe and pum beams |
| L1; bi-convex lens | Thorlabs | LB1844 | a lens to focus the probe beam at the detector, EFL 50 mm, wavelength range 350-2,000 nm |
| DM, dichroic mirror | Thorlabs | DMLP505 | a longpass dichroic mirror with a cutoff wavelength of 505 nm |
| F1; Edge filter | Andower | 500FH90-25 | a long pass filter with a cutoff wavelength of 500 nm |
| Temperature-controlled cuvette holder | Quantum Northwest | FLASH 300 |
References
- Gensch, T., Viappiani, C. Time-resolved photothermal methods: accessing time-resolved thermodynamics of photoinduced processes in chemistry and biology. Photochem. Photobiol. Sci. 2, 699-721 (2003).
- Larsen, R. W., Mikšovská, J. Time resolved thermodynamics of ligand binding to heme proteins. Coord. Chem. Rev. 251 (9-10), 1101-1127 (2007).
- Westrick, J. A., Peters, K. S. A photoacoustic calorimetric study of horse myoglobin. Bioph. Chem. 37 (1-3), 73-79 (1990).
- Belogortseva, N., Rubio, M., Terrell, W., Miksovska, J. The contribution of heme propionate groups to the conformational dynamics associated with CO photodissociation from horse heart myoglobin. J. Inorg. Biochem. 101 (7), 977-986 (2007).
- Mikšovská, J., Suquet, C., Satterlee, J. D., Larsen, R. W. Characterization of Conformational Changes Coupled to Ligand Photodissociation from the Heme Binding Domain of FixL. Biochemistry. 44 (30), 10028-10036 (2005).
- Miksovska, J., Gennis, R. B., Larsen, R. W. Photothermal studies of CO photodissociation from mixed valence Escherichia coli cytochrome bo3. FEBS Lett. 579 (14), 3014-3018 (2005).
- Losi, A., Michler, I., Gärtner, W., Braslavsky, S. E. Time-resolved Thermodynamic Changes Photoinduced in 5,12-trans-locked Bacteriorhodopsin. Evidence that Retinal Isomerization is Required for Protein Activation. Photochem. Photobiol. 72, 590-597 (2000).
- Kondoh, M., et al. Light-Induced Conformational Changes in Full-Length Arabidopsis thaliana Cryptochrome. J. Mol. Biol. 413 (1), 128-137 (2011).
- Kaplan, J. H., Ellis-Davies, G. C. Photolabile chelators for the rapid photorelease of divalent cations. Proc. Natl. Acad. Sci. U.S.A. 85 (17), 6571-6575 (1988).
- Eisenberg, H. Equation for the Refractive Index of Water. J. Chem. Phys. 43 (11), 3887-3892 (1965).
- Ellis-Davies, G. C., Kaplan, J. H., Barsotti, R. J. Laser photolysis of caged calcium: rates of calcium release by nitrophenyl-EGTA and DM-nitrophen. Biophys. J. 70, 1006-1016 (1996).
- Miksovska, J., Larsen, R. W. Structure-function relationships in metalloproteins. Methods Enzymol. 360, 302-329 (2003).
- Miksovska, J., Norstrom, J., Larsen, R. W. Thermodynamic profiles for CO photodissociation from heme model compounds: effect of proximal ligands. Inorg. Chem. 44 (4), 1006-1014 (2005).
- Dhulipala, G., Rubio, M., Michael, K., Miksovska, J. Thermodynamic profile for urea photo-release from a N-(2-nitrobenzyl) caged urea compound. Photochem. Photobiol. Sci. 8, 1157-1163 (2009).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved