A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Development of a 3D Graphene Electrode Dielectrophoretic Device
In This Article
Summary
A microdevice with high throughput potential is used to demonstrate three-dimensional (3D) dielectrophoresis (DEP) with novel materials. Graphene nanoplatelet paper and double sided tape were alternately stacked; a 700 μm micro-well was drilled transverse to the layers. DEP behavior of polystyrene beads was demonstrated in the micro-well.
Abstract
The design and fabrication of a novel 3D electrode microdevice using 50 µm thick graphene paper and 100 µm double sided tape is described. The protocol details the procedures to construct a versatile, reusable, multiple layer, laminated dielectrophoresis chamber. Specifically, six layers of 50 µm x 0.7 cm x 2 cm graphene paper and five layers of double sided tape were alternately stacked together, then clamped to a glass slide. Then a 700 μm diameter micro-well was drilled through the laminated structure using a computer-controlled micro drilling machine. Insulating properties of the tape layer between adjacent graphene layers were assured by resistance tests. Silver conductive epoxy connected alternate layers of graphene paper and formed stable connections between the graphene paper and external copper wire electrodes. The finished device was then clamped and sealed to a glass slide. The electric field gradient was modeled within the multi-layer device. Dielectrophoretic behaviors of 6 μm polystyrene beads were demonstrated in the 1 mm deep micro-well, with medium conductivities ranging from 0.0001 S/m to 1.3 S/m, and applied signal frequencies from 100 Hz to 10 MHz. Negative dielectrophoretic responses were observed in three dimensions over most of the conductivity-frequency space and cross-over frequency values are consistent with previously reported literature values. The device did not prevent AC electroosmosis and electrothermal flows, which occurred in the low and high frequency regions, respectively. The graphene paper utilized in this device is versatile and could subsequently function as a biosensor after dielectrophoretic characterizations are complete.
Introduction
Graphene is a novel material known for its high quality electronic properties and potential chemical and biosensor applications1. Graphene nanoplatelets have been used for catalyst support2,3, biosensors4, super-capacitors5, and composite-electrodes including graphene/polyaniline and silicon nanoparticle/graphene composites6-8. This manuscript describes utilization of graphene paper as electrodes in a unique three-dimensional (3D), layered microfluidic device. Graphene paper electrodes were laminated with insulative double-sided tape and a chamber drilled within which 3D AC dielectrophoresis of polystyrene beads was performed.
Dielectrophoresis (DEP) refers to the movement of polarizable particles under non-uniform electrical fields. Positive DEP (pDEP) or negative DEP (nDEP) occurs when particles are more or less polarizable than the surrounding medium, resulting in movement toward the strongest or weakest electrical field, respectively. This nonlinear electrokinetic tool has been used for separation, sorting, trapping, and identification of particles and biological cells9-15. The dielectrophoretic force experienced by a polarized particle is a function of the electric field gradient, particle radius and shape, particle dielectric properties including conductivity and permittivity, as well as the media conductivity and permittivity. In traditional two-dimensional (2D) DEP, particle movement is in the primary plane of the electric field gradient typically formed between microfabricated surface electrodes; movement in the vertical direction is negligible compared to in-plane directions in most devices. However, harnessing this third dimension of electrical field gradients for 3D DEP allows for higher sample throughput and increases the versatility to design new and improved dielectrophoretic separations in which the flow is traverse to the field gradients16,17. Other specific designs include 3D insulator-based DEP18, 3D carbon-electrode DEP13,19, and 3D electroplating DEP10. As evidenced by the research into 3D structures, such devices can be operated in continuous flow mode to achieve higher throughputs. Observation of the 3D particle movement in our layered 3D device is achieved as a function of frequency and medium conductivity via light microscopy at different focal heights.
Fatoyinbo et al. first reported DEP in a 3D laminated electrode/insulation structure using alternatively stacked 30 μm aluminum foil and 150 μm epoxy resin films20. Hubner et al. then designed similar 3D laminated electrodes with 35 μm copper tape and 118 μm polyimide adhesive21. This work borrows the 3D-well design22,23, and uniquely utilizes the convenience of 50 μm graphene paper as the conducting layers and 100 μm double-sided tape as the insulating layers, which achieved sealing and sufficient electrical shielding. Graphene paper versatility is a distinct advantage for 3D electrode microdevices because the graphene nanoplatelets have the ability to concurrently act as biosensors, which this group previously demonstrated24.
The field gradients achieved within the graphene paper/polymer laminated 3D microdevices depend on the micro-well dimensions, the graphene paper layers, and the applied electric field. Critical dimensions include the vertical electrode spacing (conducting and insulating layer thicknesses) and micro-well diameter and height (determined by layers stacked). The electric signal can be tuned via amplitude and frequency. The current device structure is for batch operation, but can be tailored to a continuous flow device. The device fabrication technique described here is suitable for developing 3D laminated electrodes with a wide variety of graphene nanoplatelet properties simply by exchanging the graphene paper utilized. Advantages of utilizing graphene paper are versatility of physical and chemical properties, reduced expense, and the graphene nanoplatelets can concurrently act as biosensors to detect a wide range of bioanalytes24. Long-term goals of high throughput 3D DEP systems are to rapidly identify cell types25-27, or achieve label-free, electrically mediated cell sorting of diseased cells from populations of healthy cells28. This paper demonstrates material optimization and device preparation and operation followed by illustration and analysis of typical results.
Protocol
1. Fabricate a Laminated Electrode/insulation 3D Structure
- For a 6 graphene layer, 5 tape layer device, cut graphene paper with a scalpel or similar razor blade and straight-edged ruler into six 0.7 cm x 1.5 cm rectangles and use scissors to cut double-sided pressure-sensitive tape into five 1.3 cm x ~5 cm stripes.
NOTE: As shown in Figure 1a, this yields a 3 ground electrode, 3 AC signal electrode device. The 7 mm conducting layer width is narrow enough to fit onto a glass slide, yet wide enough for easy drilling. The 2 mm length does not easily break upon repeated use and has sufficient room to attach copper wires. The device depth is limited by end mill depths. - Lay the first layer of graphene paper on a clean glass slide. Slowly cover one end of the graphene paper with one stripe of tape, leaving a ~2 mm margin to assure insulation between the two adjacent graphene paper layers (Figure 1b).
- Place the second layer of graphene paper on the top of the tape offset to the first layer of graphene paper (Figure 1a). Apply moderate pressure (press uniformly with thumb, ~100 N over 0.7 cm2 area) after addition of each conducting layer to ensure good sealing between layers.
- Repeat steps 1.2 and 1.3 for the remaining layers, leaving both the top and bottom layers graphene paper. Cut along the dotted line shown in Figure 1b to remove the excess tape from the device edges leaving a small ~1 mm margin to assure sealed insulation between graphene paper layers (Figure 1b).
NOTE: Double-sided tape is not utilized as the top and bottom layers to avoid collecting debris as this laminated structure is drilled, mounted on a slide, and filled with sample. - Perform a quick insulation test with a multimeter (resistance mode). Position the positive and negative probes on two different sides of the device (A and B on Figure 1c); high resistance (kilo- to mega-Ohms) indicates good insulation between layers. Remove the layered structure from the glass slide to prepare for micro-well drilling.
NOTE: A device typically fails the insulation test when adjacent graphene paper layers make contact during steps 1.2 through 1.4. Discard such devices.
2. Drill Micro-well in the Laminated Structure
- Use a computer-controlled mechanical micro-milling machine and choose an end mill with a 700 µm in diameter and 2.1 mm length of cut. Immobilize the laminated structure on the micro-milling stage using appropriate clamps (Figures 2a and b). Run the milling machine spindle at 8,600 rpm, then lower the end mill slowly into and through the center of the laminated structure. Move the rotating end mill up and down through the micro-well to smooth the inside wall.
- Choose micro-well diameters, which are constrained by available end mill diameter/length of cut aspect ratios. Ensure that the inner surface of micro-well is as vertical and clean as possible for optimal electric field gradients and light passage through the micro-well.
- Clean debris from micro-well with pressurized air. Perform another insulation test as described in 1.5.
3. Attach Electrical Leads to the Laminated Structure
- Fold two 3 cm long 32 G copper wires to a right angle at 2 cm. Mix ~1.5 ml of part A and B of silver conductive epoxy.
NOTE: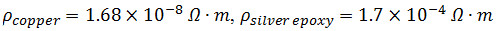
- Manually apply mixed silver epoxy to the top and the tips of all 3 graphene paper layers to assure good contact between layers on side A of the laminated structure (Figure 1c), then place the 1 cm copper wire end in the epoxy and between any two layers. Softly squeeze the layers to remove excess epoxy and assure good electrical contact. Repeat for side B of the laminated structure.
- Place the whole device in oven rack, to dry overnight 70 °C at and 1 atm.
4. Prepare Sample and Media
- Prepare isotonic media of a spectrum of conductivities using the conductivity meter, 290 mM mannitol stock solution and serial additions of isotonic phosphate buffer saline (PBS).
NOTE: A linear correlation exists between the conductivity and volume concentration of ~290 mOsm/L PBS (conducting) in ~290 mOsm/L mannitol solution (non-conducting). The video features a medium of 0.01 S/m conductivity. - Mix polystyrene beads with prepared conductivity media or e-pure water (~5 x 10-6 S/m) to a 1:50 vol:vol ratio. This protocol is easily adaptable to biological cells as well.
5. Setup Experiment and Operate Device
- Clamp the device onto a glass slide with moderate pressure (Figure 2d) using modified paper clamps or equivalent. The footings should be close enough to the micro-well to seal the laminated structure to the glass slide preventing sample leakage. The clamp should fit within the microscope stage with pressure optimized to a) prevent deformation of the laminated structure, and b) ensure the micro-well fluid does not leak. Deformation alters the well geometry and light path reducing experiment reproducibility.
- Using a micro syringe or equivalent, slowly inject ~1 µl of the sample into the micro-well and avoid introducing any bubbles. Repeat injection if necessary and use care to not damage the micro-well walls with the sharp needle. Slightly overfill the micro-well and immediately slide cover glass over the micro-well to remove excess fluid, prevent evaporation, and ensure reproducible volumes for each experiment.
NOTE: A diamond tip glasscutter works well to score and crack cover glass to size. - Secure the completed laminated microdevice to the microscope stage and attach the function generator electrode wires to the two copper leads on the device. In AxioVision (Zeiss software), click button to start the camera recording in multidimensional acquisition mode. Initiate function generator signal at a fixed time period after starting the CCD camera recording to document responses with and without the electric field applied.
NOTE: Here 100 Hz to 10 MHz frequency with a 15 Vpeak-peak signal were applied and experiments were observed at 10X magnification at 1 to 200 above the glass slide surface for 2 sec without field and ~5 min with field applied. Images were digitally saved at 1 to 5 frames per sec (fps) for further analysis. - Upon experiment completion, remove the device and dismantle the clamps. Immerse both the glass slide and device in soapy water, then rinse well. Reuse devices about 30 times with consistent performance.
6. Data Analysis and Image Processing
- Analyze image data with preferred software, such as ImageJ. Calculate velocity from the particle displacement between consecutive images at a given time step.
- Calculate experimental DEP force and field strength based on velocity to compile trends and compare with theory29.
- Measure particle velocity radially in the micro-well geometry consistent with the shape of the electric field gradient. From the micro-well edge to the center, identify 8 concentric isoelectric contours (350, 300,…50, 0 µm), which results in 7 regions.
NOTE: The time for particles to traverse the 50 µm distance was used to calculate velocity. When geometric variations necessitated it, the isoelectric contours were adjusted slightly.
Results
Dielectrophoretic experiments on 6 µm polystyrene beads were conducted in a 0.38 mm3 cylindrical micro-well. Results demonstrate that a 3D laminated graphene paper-based device can illustrate similar dielectrophoretic signatures as 3D metal foil laminated devices20,21, traditional 2D metal-electrode26,27, and 2D insulator devices25. In the following experiments, an 15 Vpeak-peak AC signal was applied and frequency...
Discussion
This manuscript details protocols for fabricating a novel 6 graphene layer and 5 tape layer microdevice. Further, device operation is illustrated via observed DEP behaviors of 6.08 µm polystyrene beads along with a unique, geometrically relevant particle velocity analysis approach. This versatile approach to construct nonlinear electrokinetic devices is less costly than electrode and fluidic layer microfabrication techniques, while yielding equally reliable results.
Further, this novel 3D...
Disclosures
The authors have no conflicts to disclose.
Acknowledgements
Thanks to XG Sciences for generous donations of graphene paper. Thanks to Dr. C. Friedrich for generously letting us use the micro-drilling equipment. A special thanks is extended to Tayloria Adams for narrating the video.
Materials
| Name | Company | Catalog Number | Comments |
| Polystyrene Beads | Spherotech, Inc. | PP-60-10 | 6.08 μm diameter |
| Graphene paper | XG Sciences, Inc. | XG Leaf B-072 | |
| Double sided tape | 3M | N/A | 136 office tape |
| Silver conductive epoxy | MG chemicals | 8331-14G | Part A & B included |
| Mannitol | Sigma Aldrich | 091M0020V | |
| Phosphate buffer saline | OmniPur | 0381C490 | |
| Microscope (CCD Camera) | Zeiss | Axiovert 200M | |
| Function/waveform generator | Agilent | 33250A | |
| Syringe | Hamilton | 84505 | |
| Paper Clamp | ADAMS | 3300-50-3848 | |
| Oven | Fisher Scientific | 280A | |
| Multimeter | OMEGA | HHM25 | |
| Micro-milling machine | AEROTECH | ABL1500 stages/A3200 Npaq controller | |
| End mill | ULTRATOOL | 708473 | |
| AxioVision | Zeiss | Version 4.8 |
References
- Geim, A. K., Novoselov, K. S. The rise of graphene. Nature Materials. 6 (3), 183-191 (2007).
- Jafri, R. I., Rajalakshmi, N., Ramaprabhu, S. Nitrogen doped graphene nanoplatelets as catalyst support for oxygen reduction reaction in proton exchange membrane fuel cell. Journal of Materials Chemistry. 20 (34), 7114-7117 (2010).
- Kavan, L., Yum, J. H., Gratzel, M. Graphene Nanoplatelets Outperforming Platinum as the Electrocatalyst in Co-Bipyridine-Mediated Dye-Sensitized Solar Cells. Nano Letters. 11 (12), 5501-5506 (2011).
- Aravind, S. S. J., Baby, A. T. T., Arockiadoss, T., Rakhi, R. B., Ramaprabhu, S. A cholesterol biosensor based on gold nanoparticles decorated functionalized graphene nanoplatelets. Thin Solid Films. 519 (16), 5667-5672 (2011).
- Si, P., Ding, S. J., Lou, X. W., Kim, D. H. An electrochemically formed three-dimensional structure of polypyrrole/graphene nanoplatelets for high-performance supercapacitors. Rsc Advances. 1 (7), 1271-1278 (2011).
- Wang, D. -. W., et al. Fabrication of Graphene/Polyaniline Composite Paper via In Situ Anodic Electropolymerization for High-Performance Flexible Electrode. ACS Nano. 3 (7), 1745-1752 (2009).
- Lee, J. K., Smith, K. B., Hayner, C. M., Kung, H. H. Silicon nanoparticles-graphene paper composites for Li ion battery anodes. Chem Commun (Camb). 46 (12), 2025-2027 (2010).
- Kavan, L., Yum, J. H., Gratzel, M. Optically Transparent Cathode for Dye-Sensitized Solar Cells Based on Graphene Nanoplatelets. ACS Nano. 5 (1), 165-172 (2011).
- Martinez-Duarte, R. Microfabrication technologies in dielectrophoresis applications--a review. Electrophoresis. 33 (21), 3110-3132 (2012).
- Yamamoto, M., et al. Patterning with particles using three-dimensional interdigitated array electrodes with negative dielectrophoresis and its application to simple immunosensing. Electrochimica Acta. 82, 35-42 (2012).
- Doh, I., Kim, Y., Cho, Y. H. A particle trapping chip using the wide and uniform slit formed by a deformable membrane with air bubble plugs. Current Applied Physics. 13 (5), 902-906 (2013).
- Lin, S. C., Lu, J. C., Sung, Y. L., Lin, C. T., Tung, Y. C. A low sample volume particle separation device with electrokinetic pumping based on circular travelling-wave electroosmosis. Lab on a Chip. 13 (15), 3082-3089 (2013).
- Martinez-Duarte, R., Camacho-Alanis, F., Renaud, P., Ros, A. Dielectrophoresis of lambda-DNA using 3D carbon electrodes. Electrophoresis. 34 (7), 1113-1122 (2013).
- Yang, S. M., Tseng, S. Y., Chen, H. P., Hsu, L., Liu, C. H. Cell patterning via diffraction-induced optoelectronic dielectrophoresis force on an organic photoconductive chip. Lab on a Chip. 13 (19), 3893-3902 (2013).
- Srivastava, S. K., Gencoglu, A., Minerick, A. R. DC insulator dielectrophoretic applications in microdevice technology: a review. Anal Bioanal Chem. 399 (1), 301-321 (2011).
- Liao, S. H., Cheng, I. F., Chang, H. C. Precisely sized separation of multiple particles based on the dielectrophoresis gradient in the z-direction. Microfluidics and Nanofluidics. 12 (1-4), 1-4 (2012).
- Bajaj, P., Marchwiany, D., Duarte, C., Bashir, R. Patterned three-dimensional encapsulation of embryonic stem cells using dielectrophoresis and stereolithography. Adv Healthc Mater. 2 (3), 450-458 (2013).
- Braff, W. A., Pignier, A., Buie, C. R. High sensitivity three-dimensional insulator-based dielectrophoresis. Lab Chip. 12 (7), 1327-1331 (2012).
- Martinez-Duarte, R., Gorkin 3rd, R. A., Abi-Samra, K., Madou, M. J. The integration of 3D carbon-electrode dielectrophoresis on a CD-like centrifugal microfluidic platform. Lab Chip. 10 (8), 1030-1043 (2010).
- Fatoyinbo, H. O., Kamchis, D., Whattingham, R., Ogin, S. L., Hughes, M. P. A high-throughput 3-D composite dielectrophoretic separator. Ieee Transactions on Biomedical Engineering. 52 (7), 1347-1349 (2005).
- Hubner, Y., Hoettges, K. F., Kass, G. E. N., Ogin, S. L., Hughes, M. P. Parallel measurements of drug actions on Erythrocytes by dielectrophoresis, using a three-dimensional electrode design. Iee Proceedings-Nanobiotechnology. 152 (4), 150-154 (2005).
- Abdul Razak, M. A., Hoettges, K. F., Fatoyinbo, H. O., Labeed, F. H., Hughes, M. P. Efficient dielectrophoretic cell enrichment using a dielectrophoresis-well based system. Biomicrofluidics. 7 (6), (2013).
- Hughes, M. P. . O. S., Hoettges, K. F., Wattingham, R. . Device for Dielectrophoretic Manipulation of Particles. , (2005).
- Heldt, C. L., et al. Stacked graphene nanoplatelet paper sensor for protein detection. . Sensors and Actuators B-Chemica. 181, 92-98 (2013).
- Srivastava, S. K., Artemiou, A., Minerick, A. R. Direct current insulator-based dielectrophoretic characterization of erythrocytes: ABO-Rh human blood typing. Electrophoresis. 32 (18), 2530-2540 (2011).
- Leonard, K. M., Minerick, A. R. Explorations of ABO-Rh antigen expressions on erythrocyte dielectrophoresis: Changes in cross-over frequency. Electrophoresis. 32 (18), 2512-2522 (2011).
- Srivastava, S. K., Daggolu, P. R., Burgess, S. C., Minerick, A. R. Dielectrophoretic characterization of erythrocytes: Positive ABO blood types. Electrophoresis. 29 (24), 5033-5046 (2008).
- Minerick, A. R. The rapidly growing field of micro and nanotechnology to measure living cells. AIChE Journal. 54 (9), 2230-2237 (2008).
- Garza-Garcia, L. D., Perez-Gonzalez, V. H., Perez-Sanchez, O. A., Lapizco-Encinas, B. H. Electrokinetic Mobilities Characterization and Rapid Detection of Microorganisms in Glass Microchannels. Chemical Engineering & Technology. 34 (3), 371-378 (2011).
- Lopez-de la Fuente, M. S., et al. An electric stimulation system for electrokinetic particle manipulation in microfluidic devices. Rev Sci Instrum. 84 (3), (2013).
- Chen, D. F., Du, H., Li, W. H. A 3D paired microelectrode array for accumulation and separation of microparticles. Journal of Micromechanics and Microengineering. 16 (7), 1162-1169 (2006).
- Chu, H., Doh, I., Cho, Y. H. A three-dimensional (3D) particle focusing channel using the positive dielectrophoresis (pDEP) guided by a dielectric structure between two planar electrodes. Lab on a Chip. 9 (5), 686-691 (2009).
- Millet, L. J., Park, K., Watkins, N. N., Hsia, K. J., Bashir, R. Separating beads and cells in multi-channel microfluidic devices using dielectrophoresis and laminar flow. J Vis Exp. , (2011).
- Weiss, N. G., et al. Dielectrophoretic mobility determination in DC insulator-based dielectrophoresis. Electrophoresis. 32 (17), 2292-2297 (2011).
- Auerswald, J., Knapp, H. F. Quantitative assessment of dielectrophoresis as a micro fluidic retention and separation technique for beads and human blood erythrocytes. Microelectronic Engineering. 67-8, 879-886 (2003).
- Park, S., Zhang, Y., Wang, T. H., Yang, S. Continuous dielectrophoretic bacterial separation and concentration from physiological media of high conductivity. Lab on a Chip. 11 (17), 2893-2900 (2011).
- Sun, T., Holmes, D., Gawad, S., Green, N. G., Morgan, H. High speed multi-frequency impedance analysis of single particles in a microfluidic cytometer using maximum length sequences. Lab on a Chip. 7 (8), 1034-1040 (2007).
- Hughes, M. P., Morgan, H. Dielectrophoretic Characterization and Separation of Antibody-Coated Submicrometer Latex Spheres. Analytical Chemistry. 71 (16), 3441-3445 (1999).
- Liang, W. F., et al. Simultaneous separation and concentration of micro- and nano-particles by optically induced electrokinetics. Sensors and Actuators a-Physical. 193, 103-111 (2013).
- White, C. M., Holland, L. A., Famouri, P. Application of capillary electrophoresis to predict crossover frequency of polystyrene particles in dielectrophoresis. Electrophoresis. 31 (15), 2664-2671 (2010).
- Wu, J., Ben, Y. X., Battigelli, D., Chang, H. C. Long-range AC electroosmotic trapping and detection of bioparticles. Industrial & Engineering Chemistry Research. 44 (8), 2815-2822 (2005).
- Zhou, H., White, L. R., Tilton, R. D. Lateral separation of colloids or cells by dielectrophoresis augmented by AC electroosmosis. J Colloid Interface Sci. 285 (1), 179-191 (2005).
- Green, N. G., Ramos, A., Gonzalez, A., Morgan, H., Castellanos, A. Fluid flow induced by nonuniform ac electric fields in electrolytes on microelectrodes I. Experimental measurements. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 61 (4 Pt B), 4011-4018 (2000).
- Green, N. G., Ramos, A., Gonzalez, A., Castellanos, A., Morgan, H. Electrothermally induced fluid flow on microelectrodes. Journal of Electrostatics. 53 (2), 71-87 (2001).
- Gonzalez, A., Ramos, A., Morgan, H., Green, N. G., Castellanos, A. Electrothermal flows generated by alternating and rotating electric fields in microsystems. Journal of Fluid Mechanics. 564, 415-433 (2006).
- Park, S., Koklu, M., Beskok, A. Particle trapping in high-conductivity media with electrothermally enhanced negative dielectrophoresis. Anal Chem. 81 (6), 2303-2310 (2009).
- Sin, M. L., Gau, V., Liao, J. C., Wong, P. K. Electrothermal Fluid Manipulation of High-Conductivity Samples for Laboratory Automation Applications. JALA Charlottesv Va. 15 (6), 426-432 (2010).
- Liao, S. -. H., Cheng, I. F., Chang, H. -. C. Precisely sized separation of multiple particles based on the dielectrophoresis gradient in the z-direction. Microfluidics and Nanofluidics. 12 (1-4), 201-211 (2012).
- Gencoglu, A., Minerick, A. Chemical and morphological changes on platinum microelectrode surfaces in AC and DC fields with biological buffer solutions. Lab on a Chip. 9 (13), 1866-1873 (2009).
- Bocchi, M., et al. Dielectrophoretic trapping in microwells for manipulation of single cells and small aggregates of particles. Biosensors & Bioelectronics. 24 (5), 1177-1183 (2009).
- Li, P., Stratton, Z. S., Dao, M., Ritz, J., Huang, T. J. Probing circulating tumor cells in microfluidics. Lab on a Chip. , (2013).
- Rimmele, T., Kellum, J. A. Clinical review: Blood purification for sepsis. Critical Care. 15 (1), (2011).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved