Method Article
Methods to Assess Subcellular Compartments of Muscle in C. elegans
In This Article
Summary
Skeletal muscle is essential for locomotion and is the bodies’ main protein store. Muscle health measurements within C. elegans are described. Prospective changes to muscle structure and function are assessed using localized GFP and cationic dyes.
Abstract
Muscle is a dynamic tissue that responds to changes in nutrition, exercise, and disease state. The loss of muscle mass and function with disease and age are significant public health burdens. We currently understand little about the genetic regulation of muscle health with disease or age. The nematode C. elegans is an established model for understanding the genomic regulation of biological processes of interest. This worm’s body wall muscles display a large degree of homology with the muscles of higher metazoan species. Since C. elegans is a transparent organism, the localization of GFP to mitochondria and sarcomeres allows visualization of these structures in vivo. Similarly, feeding animals cationic dyes, which accumulate based on the existence of a mitochondrial membrane potential, allows the assessment of mitochondrial function in vivo. These methods, as well as assessment of muscle protein homeostasis, are combined with assessment of whole animal muscle function, in the form of movement assays, to allow correlation of sub-cellular defects with functional measures of muscle performance. Thus, C. elegans provides a powerful platform with which to assess the impact of mutations, gene knockdown, and/or chemical compounds upon muscle structure and function. Lastly, as GFP, cationic dyes, and movement assays are assessed non-invasively, prospective studies of muscle structure and function can be conducted across the whole life course and this at present cannot be easily investigated in vivo in any other organism.
Introduction
Muscle is a multifunctional tissue, well appreciated for its role in locomotion, postural support and metabolism. The development and maintenance of healthy muscle requires the coordination of multiple cellular processes and components. Either through damage or disease, dysregulation can occur, resulting in altered muscle structure and/or function. Age-associated decline in muscle function presents a significant burden for health care budgets in the western world, since muscle mass1,2 and particularly muscle strength and endurance3-5 are negatively correlated with mortality. The measurement of aspects related to deteriorating muscle function such as altered mitochondrial function or sarcomere disruption, can allow greater understanding of the mechanisms underpinning overall muscle development and health.
Caenorhabditis elegans (C. elegans) is a microscopic nematode best known because it was the first multicellular organism to have its entire genome sequenced6, the first to have genes silenced using RNAi7, and the only metazoan to have its entire cell lineage8 and neuroanatomy9 determined. C. elegans was first proposed as a model for the study of the genetic control of behavior in 1974 by Sydney Brenner10 and the importance of this and subsequent work was recognized with the first of three Nobel Prizes awarded to C. elegans researchers. Approximately 35% of C. elegans genes have identified orthologues in humans, with C. elegans being a key organism used to understand the genetic control of muscle development11. Almost every gene in the C. elegans genome can be knocked down through RNAi7,12. Thus, by knocking down genes in fully developed muscle, genetic components contributing to the regulation of muscle health can be investigated with relative simplicity13.
The combined ability to use RNAi, mutants, and chemical compounds makes C. elegans a premier system for exploring the genetic regulation7,14,15 of muscle structure and function with age16 and in disease models17. The impact of gene knockdown, mutation, and/or exposure to chemical compounds upon muscle structure and/or function can be measured through multiple aspects. Here we briefly describe simple techniques for assessing C. elegans muscle structure and function, many of which can be used in prospective studies of muscle health.
The development of green fluorescent protein (GFP)18 has enabled visualization of both the locality of specific proteins and the general structure of organelles in C. elegans. Here we explain how GFP expressed specifically within body wall muscle mitochondria, nuclei, or sarcomeres can be used to determine if dystrophy of these sub-cellular structures is present. As structure is not always a reliable surrogate for function, we also explain how the use of cationic dyes in vivo allows assessment of impaired mitochondrial membrane potential within muscle. When the dyes are used in combination with GFP, they permit the study of architecture and function in a temporal manner throughout lifespan. Lastly, we also explain how protein homeostasis within muscle can be assessed and how all of these measures can be used to correlate changes in whole animal muscle health via use of movement assays. These techniques, combined with gene knockdown, mutations, and/or chemical compounds provide a powerful arsenal for studying how specific genes or compounds influence muscle health in vivo. The parallels in structure, metabolism and gross function of muscle between C. elegans and mammals mean C. elegans is an outstanding model organism for understanding the regulation of muscle in higher organisms, including humans.
Protocol
1. Strains, Culture, and Microscopy
- Handle and maintain strains of C. elegans as previously described19. Strains are available from the Caenorhabditis Genetics Center (CGC).
NOTE: The strains used in these experiments were CB5600 (ccIs4251 (Pmyo-3::Ngfp-lacZ; Pmyo-3::Mtgfp) I; him-8 (e1489) IV) with GFP fusion proteins localized to mitochondria and nuclei; PJ727 (jls01 (myo-3::GFP, rol-6 (su1006)); unc-54::lacZ V) with GFP fusion proteins localized to the contractile apparatus and PD55 (ccIs55 (sup-7(st5); unc-54::lacZ) V) with β-galactosidase fusion proteins localized to the cytosol. - Complete RNAi experiments as previously described for multigenerational RNAi experiments13 and obtain RNAi bacteria from the sequence verified ORF-RNAi library constructed by Marc Vidal et al.12.
- Construct strains containing ccIs4251, jls01 or ccIs55 using standard techniques20. Use these transgenes to create a strain to assess mitochondrial network structure, myosin organization or the state of proteostasis, respectively. Cross the strains into a mutant where looking at one of these sub-cellular compartments in every animal is desired.
- Use the PtdIns-3-kinase inhibitor, LY-294002 (LY) as previously described21. Briefly, add LY-294002 at a final concentration of 160 µM on top of dry OP50-seeded NGM plates.
- Capture all images using a GFP filter for GFP-based experiments and a microscope with a triple-pass filter which allows imaging of red, green and blue channels simultaneously for assessing in vivo membrane potential with C32H32Cl2N2O, commonly marketed as MitoTracker Red CMXRos. Conduct image analysis and figure preparation in image processing software.
2. Synchronization of L1 Larval Animals Using Gravity Flotation
- A minimum of 1 day before needed, make M9 buffer - 22.04 mM KH2PO4, 42.27 mM Na2HPO4, 85.56 mM NaCl (final concentrations) , and sterilize by autoclaving. Once cooled to 45 °C, add sterile MgSO4 to 1 mM after autoclaving to prevent precipitation22. Allow the M9 to cool at 4 °C and aliquot into 50 ml tubes.
- On the day of the experiment, add 3 ml M9 buffer to a single 9 cm plate with high numbers of L1 larvae. Wash the plate repeatedly by aspirating the M9 liquid and pipetting against the surface of the agar with the plate held at an angle.
- Wash the L1’s from the plate and then transfer the M9 to a 10 ml test tube using a pipette.
- Leave for 2.5 min to allow the larger adult and later larval stage animals to fall to the bottom of the test tube and the smaller L1 animals remain near the top of the M9 buffer.
- Remove the top 500 µl of M9 in the test tube using a pipette. Then pipette the M9 containing L1 larvae to a new NGM plate seeded with 500 µl OP50 under a dissecting scope.
- Typically, aim to transfer ~200-300 L1 larvae per plate. Monitor plates daily from adulthood and, if necessary, pick animals on to fresh OP50 NGM plates to prevent OP50 food source being consumed.
NOTE: This is usually 1-3 drops of M9 using a P1000 pipette, although this varies largely depending on the number of L1’s on the original plate. - If prospective studies in individual animals are desired, once animals reach adulthood, pick individual animals to separate seeded NGM plates. Transfer animals every 24–48 hr to separate from larval animals.
3. Movement Assays19
- A minimum of 2 hr before commencing experimentation, remove a 50 ml aliquot of M9 and allow to equilibrate to room temperature.
- Pick a single adult animal into 50 µl M9 buffer on a microscope slide.
- Count the number of left and rightward body bends in 10 sec, where one leftward and one rightward bend is equal to one stroke.
- Repeat the count of body strokes to give a total of at least 5 measurements from which an average can be taken. Multiply these figures by six to give the movement rate per min.
- Using a pipette, transfer the animal back to the petri dish. This method enables temporal measurement of movement throughout the entire lifespan in C. elegans.
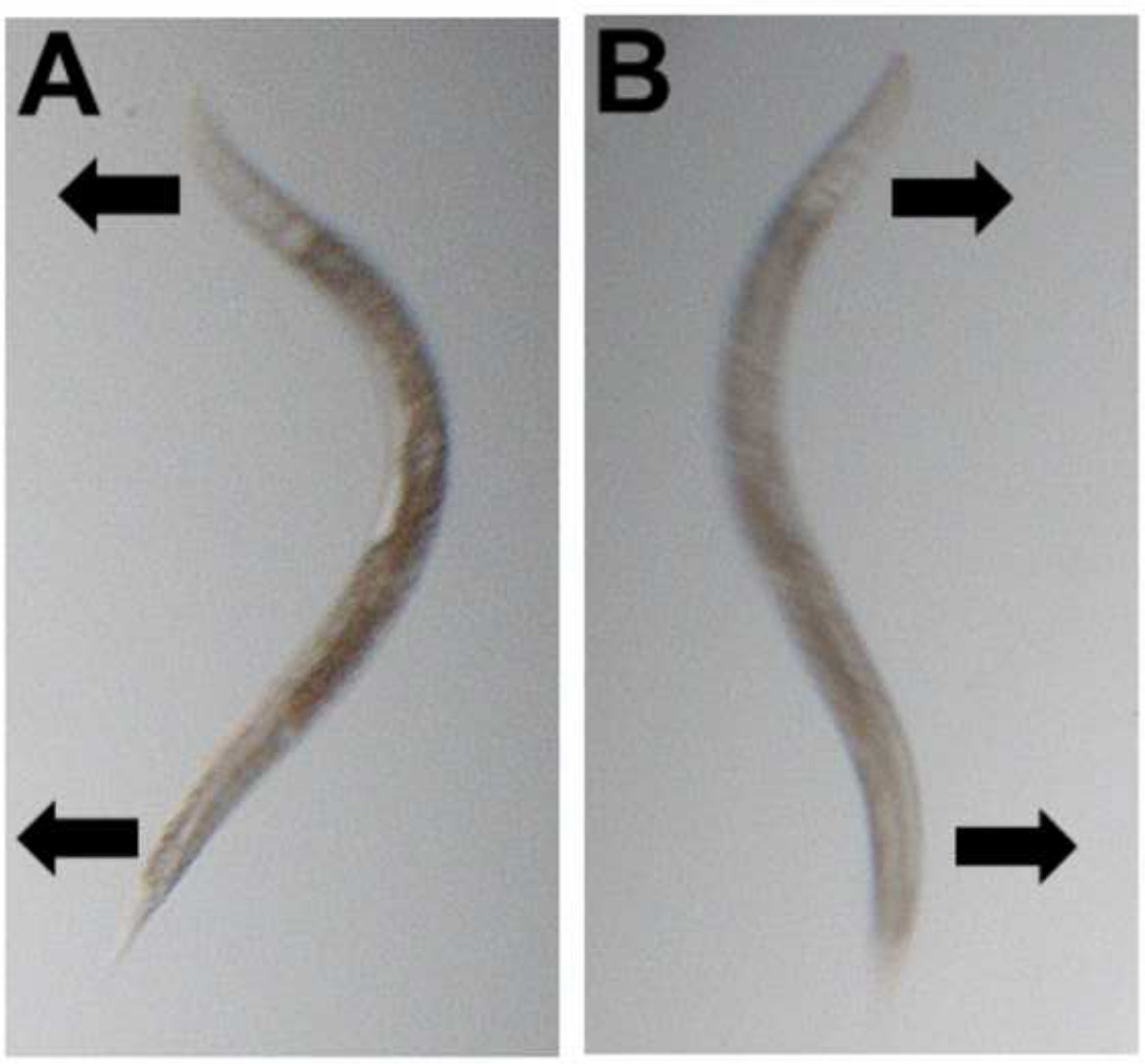
Figure 1: Movement assays. Once worms are picked into buffer, one leftward (A) and one rightward (B) bend are added together to give one complete movement (stroke).
4. Imaging of Mitochondrial Networks and Sarcomeres in Body Wall Muscle
- NOTE: The same protocol applies for imaging of both the mitochondria and sarcomeres.
- Synchronize worms as previously described (Section 1) and grow for 48-60 hr at 20 °C when they will reach young adulthood.
- Pick 20 worms (representative of the plate) from the plates into 20 µl M9 on a microscope slide.
- Apply a cover slip and proceed to image using a fluorescent microscope under a GFP filter (460-500 nm, blue light excitation) at 40X magnification.
5. Assessing Membrane Potential by Using an In Vivo Accumulation Assay with MitoTracker Red CMXRos (C32H32Cl2N2O)
- Synchronize CB5600 (Section 1), all labeled with GFP in the mitochondria of muscles, and grow to early adulthood on NGM containing OP50 for 48-60 hr at 20 °C.
- Add 100 µl DMSO to the 50 µg aliquot of C32H32Cl2N2O to make a stock solution of 940.692 µM.
- Take 1 µl of the 940.692 µM stock solution and resuspend into 199 µl M9 to create a working solution of 4.70 µM (4.70346 µM). Prepare this in brown 1.5 ml tubes because C32H32Cl2N2O is photosensitive.
- Prepare 20 µl aliquots of the 4.70 µM working solution (20 adult worms per 20 µl aliquot).
- Pick 20 adult animals into 20 µl of 4.70 µM C32H32Cl2N2O in the brown 1.5 ml tubes. Incubate at 20 °C for 1 hr.
- Following the 1 hr incubation, add 1 ml M9 to the brown 1.5 ml tubes containing the worms, centrifuge at 2,000 x g for 10 sec and then remove the supernatant. Repeat this wash step before resuspending the worm pellet and transferring the remaining 20 µl (containing the worms) to a slide for imaging.
- Take images of the mitochondria with 40X magnification using a triple pass filter which allows visualization of both the red, blue and green channels simultaneously. Imaging using the triple-pass filter shows co-localization of C32H32Cl2N2O with GFP localized to mitochondria appearing as orange.
- Having taken an image of the orange mitochondria, photo-bleach the animal for 10 sec using light of wavelength 540/25 nm on maximum settings with all filters removed. This will bleach the red within the mitochondria and reveal the mitochondrial GFP alone to confirm colocalization in muscle mitochondria.
6. Assessment of Muscle Protein Degradation in C. elegans
- Synchronize PD55s (or any strain containing the lacZ transgene) on to NGM plates seeded with OP50 (Section 1). Grow to young adulthood for 48-60 hr at 20 °C.
- Pick 20 adult worms into 20 µl ddH2O on a microscope slide. Be careful to try and pick only the worm and not any bacteria with the pick. Label the microscope slide in pencil.
- Place in the desiccator for 30 min to dry and crack the cuticle of the animals. Ensure a tight seal on the desiccator using grease around the seal.
- Check the desiccation has been effective under a dissecting scope (Figure 2).
- Submerge the microscope slide in ice-cold acetone for 3.5 min then leave the microscope slide on paper towel at room temperature for 15 min to allow the acetone on the slide to evaporate. At this point thaw the X-gal and oxidation buffer and allow equilibrating to room temperature.
- Add 2 µl X-gal to 100 µl oxidation buffer and vortex gently to ensure this is homogenous. This is the X-gal/oxidation buffer master mix.
- Add 20 µl of the X-gal/oxidation buffer master mix to each original area of 20 worms. Inspect under a dissecting scope to ensure the master mix completely covers all animals totally. Gently tilt the microscope slide to move the master mix over areas where animals are not covered.
- Place the microscope slide in a humidity chamber for 1-2.5 hr at 20 °C. Image animals when a dark blue color is present in the body wall muscles of control animals. Assess control animals under a dissecting scope every 15 min to determine when animals should be imaged.
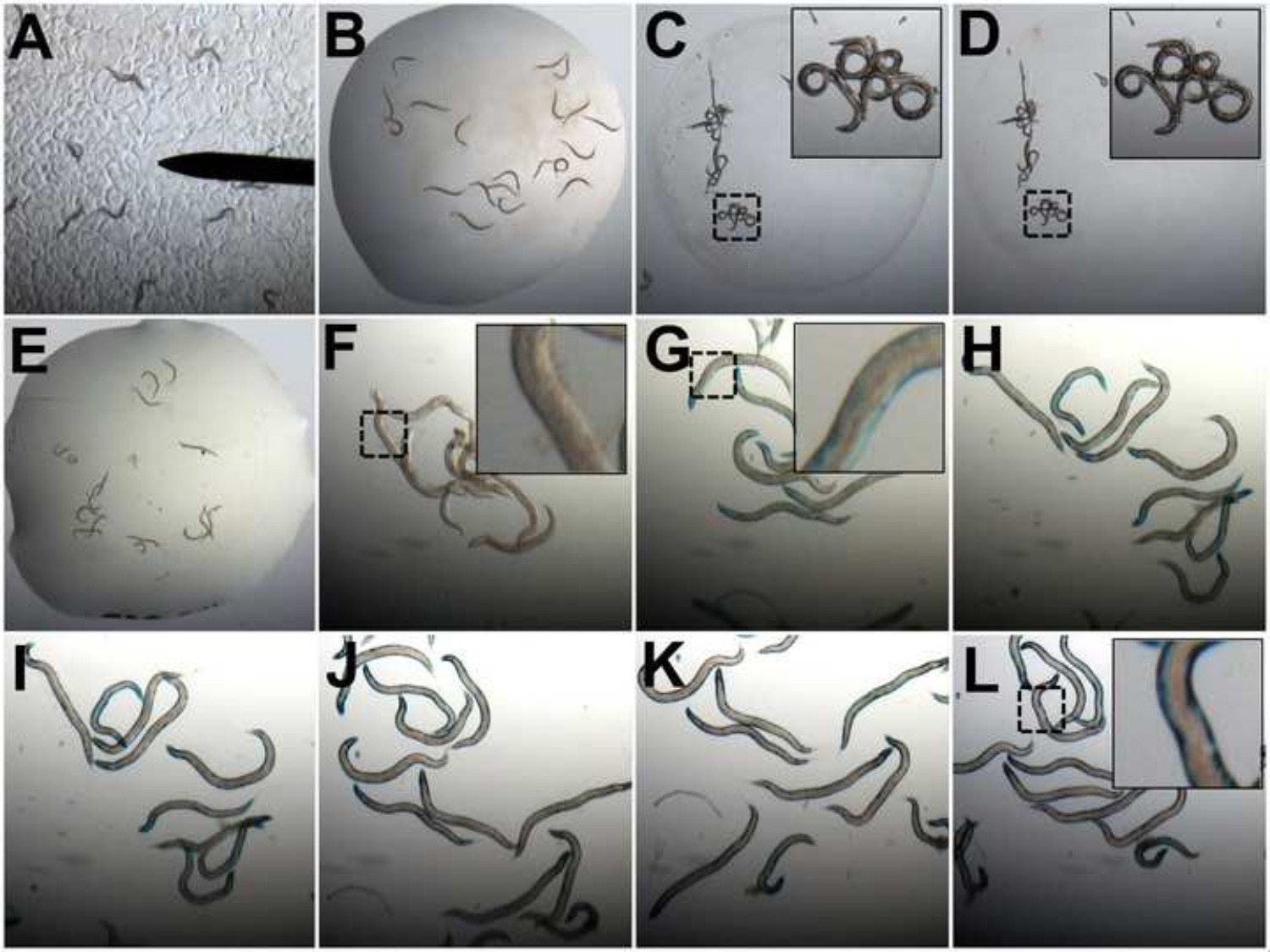
Figure 2: Assessment of muscle protein degradation using β-galactosidase assays. (A) Animals containing a lacZ transgene are picked from the Petri dishes to 20 µl ddH2O on a microscopy slide (B). Animals are desiccated and become fractured (C). Animals are then washed in acetone (D). Once acetone evaporates the X-gal/oxidation buffer master mix is added (E). The development of blue color in control animals is assessed at 15 min intervals (F-L) from t = 0 min (F) until a dark blue color is obtained across the length of the animals (L). Images of worms are taken at 2.5X magnification in A-E and 8X in F-L. Zooms in C, D, F, G, and L are 4X the original magnification.
Results
Movement Assays to Quantify Movement Defects
Movement decline is a good predictor of mortality in C. elegans16, with decreased rates indicating problems with the neuromuscular system. Changes in movement, as assessed by movement assays, can result from RNAi against a specific gene or drug treatment (Figures 3-5) or in mutant animals13,23. A decline in movement can result from altered development for example following developmental knock down of electron transport genes encoding complex I, III, IV or V24. Additionally, specific interventions can be tested on only adult C. elegans in order to investigate the effect on post-developmental physiology. For example, animals treated with RNAi against unc-112, which encodes the C. elegans orthologue of Kindlin-1 which is localized to integrin containing muscle attachment complexes (Figures 3 and 4), or gas-1, which encodes a component of complex I of the electron transport chain (Figure 4), display a progressive reduction in movement versus control from 48-72 hr post-adulthood. This may be indicative of problems with muscle physiology. Similarly, a loss of movement from 24 - 72 hr is observed in response to treatment of normally developed adults with treatment with the PI3K inhibitor LY-294002 (Figure 5). It is also possible to test the effect of a second RNAi treatment, mutation, or a drug in restoring movement in animals displaying reduced movement in response to an initial intervention. For example, unc-112 mutants display a quantifiable reduction in movement compared to wild type animals that is attenuated in the presence of a second mutation in dim-113. Therefore, movement assays can identify both interventions that alter neuromuscular development and/or function as well as ones that improve and/or restore neuromuscular function.
Assessment of Sarcomere Disruption
Having identified a movement defect, it is often desirable to determine if the cause of the movement defect can be explained by problems within nerves, muscle, or both. Sarcomeres are the multi-protein complexes within muscle that allow for contraction and thus force generation and movement. By utilizing animals expressing myosin GFP fusion proteins localized to the contractile apparatus, the extent of disruption to myosin filaments and sarcomeres can be observed relatively non-invasively and prospectively in individual animals through development and/or adulthood. Wild-type animals display arrayed sarcomeres that appear as multiple green parallel straight lines within each body-wall muscle (Figure 3). Disruption to these normal arrays may be relatively minor with arrayed sarcomeres appearing to merge rather than remaining in parallel. This phenotype, visible with unc-112 RNAi at t = 24 hr or 48 hr (Figure 3), is comparable to Z-line streaming of actin attachment sites in mammalian muscle. Similarly, small portions of the arrays can collapse or they can disappear completely such as treatment with unc-112 RNAi at t = 48 hr or t = 72 hr (Figure 3). The presence of sarcomere defects within the muscle of animals that display a movement defect (Figure 3) is sufficient to account for the movement defect but does not necessarily rule out other problems with muscle, nerve, and/or other tissues in response to an intervention.
Assessment of Mitochondrial Network Structure
In the presence of a movement defect it is sometimes desirable to examine muscle for other defects that may cause or contribute to the movement defect. Mitochondria provide the bulk of cellular energy and declines in movement may be the result of reduced ATP provision, reducing the energy available for muscle contraction. Thus the structure of mitochondria may be examined for abnormalities. It is important not to anaesthetize animals with sodium azide as it induces rapid mitochondrial fragmentation; animals in these experiments were immobilized by the pressure applied by the cover slip. Within wild-type C. elegans body wall muscle, mitochondria are contained within organized networks (Figure 4) and mitochondria exist and function as a reticulum25. Disruption of the network of mitochondria presents as fragmentation of the network (gas-1 RNAi, Figure 4B)26. Fragmentation may become severe enough that no semblance of organized networks remains such as treatment with unc-112 RNAi (Figure 4B and C)13. As with disruptions in sarcomere structure, defects in muscle mitochondrial structure in animals that display a movement defect may be sufficient to account for the movement defect but this does not necessarily rule out other problems with muscle, nerve, and/or other tissues. For example, unc-112 mutants13 and treatment with unc-112 RNAi (Figures 3 and 4) display both disrupted sarcomeres and disrupted mitochondrial structure.
Assessment of Mitochondrial Function In Vivo
The mitochondrial membrane potential determines the capacity of mitochondria to create proton motive force and generate ATP27, therefore the ability to assess mitochondrial membrane potential in vivo indicates the capacity of that animal to produce ATP. As mitochondrial structural disruptions may or may not alter mitochondrial functional capacity, it may be desirable to assess mitochondrial function in animals displaying altered mitochondrial structure. C32H32Cl2N2O accumulates in mitochondria, of all cell types, where a membrane potential is present and stains the mitochondria28 (Figure 4C). To specifically assess mitochondrial function in muscle, one can use C32H32Cl2N2O and GFP labeled muscle mitochondria to demonstrate the effects of an intervention specifically upon muscle mitochondria (Figure 4C). For example, the loss of mitochondrial membrane potential following treatment with unc-112 RNAi prevents C32H32Cl2N2O from entering the matrix. The mitochondria therefore show little, if any red staining of the mitochondria in addition to the GFP labeling of mitochondria (Figure 4C). Photo-bleaching can also be used to confirm that the observed mitochondria are specifically those in the muscle (Figure 4C). Identification of a reduced capacity to produce ATP is sufficient to account for an observed movement defect but does not rule out other problems within muscle, nerve, and/or other tissues. Regardless, as reduced ATP production capacity is associated with disease states in humans29 and ageing30, identification of such a defect in response to an intervention provides a platform for screening for compounds and/or RNAi treatments that improve ATP production capacity and these may then be further explored for therapeutic potential.
Assessment of Protein Degradation
In the presence of a movement defect and normal sarcomeres and mitochondria it is sometimes desirable to examine muscle for other defects that may cause or contribute to the movement defect. The ability to maintain protein homeostasis is a signature of normal health, whereas a reduction in protein synthesis and/or an increase in protein degradation is a consequence of ageing31 and multiple disease states32,33. Therefore it may be desirable to examine muscles of animals with a movement defect for altered protein homeostasis. This may be accomplished via assessment of levels of cytosolic muscle proteins. The use of transgenically encoded proteins allows assessment of muscle specific proteostasis. For example, use of animals containing β-galactosidase, fused to an N-terminal portion of myosin, that is synthesized under the control of a myosin promoter and enhancer allows assessment of cytosolic muscle protein content19. The presence of blue stain in wild-type adults demonstrates the presence of active β-galactosidase and the absence of pathologic cytosolic protein degradation. A loss of staining in response to treatment suggests pathologic protein degradation is occurring (Figure 5), as β-galactosidase is stable for 72 - 96 hr post adulthood in wild-type adults13,14,19. For example, untreated wild-type animals display an intense blue stain 72 hr after adulthood, whereas LY-249002 treated animals display a lack of stain (Figure 5). As with defects in sarcomere structure and mitochondrial function, the presence of pathologic muscle protein degradation is sufficient to account for an observed movement defect but does not necessarily rule out other problems with muscle, nerve, and/or other tissues.
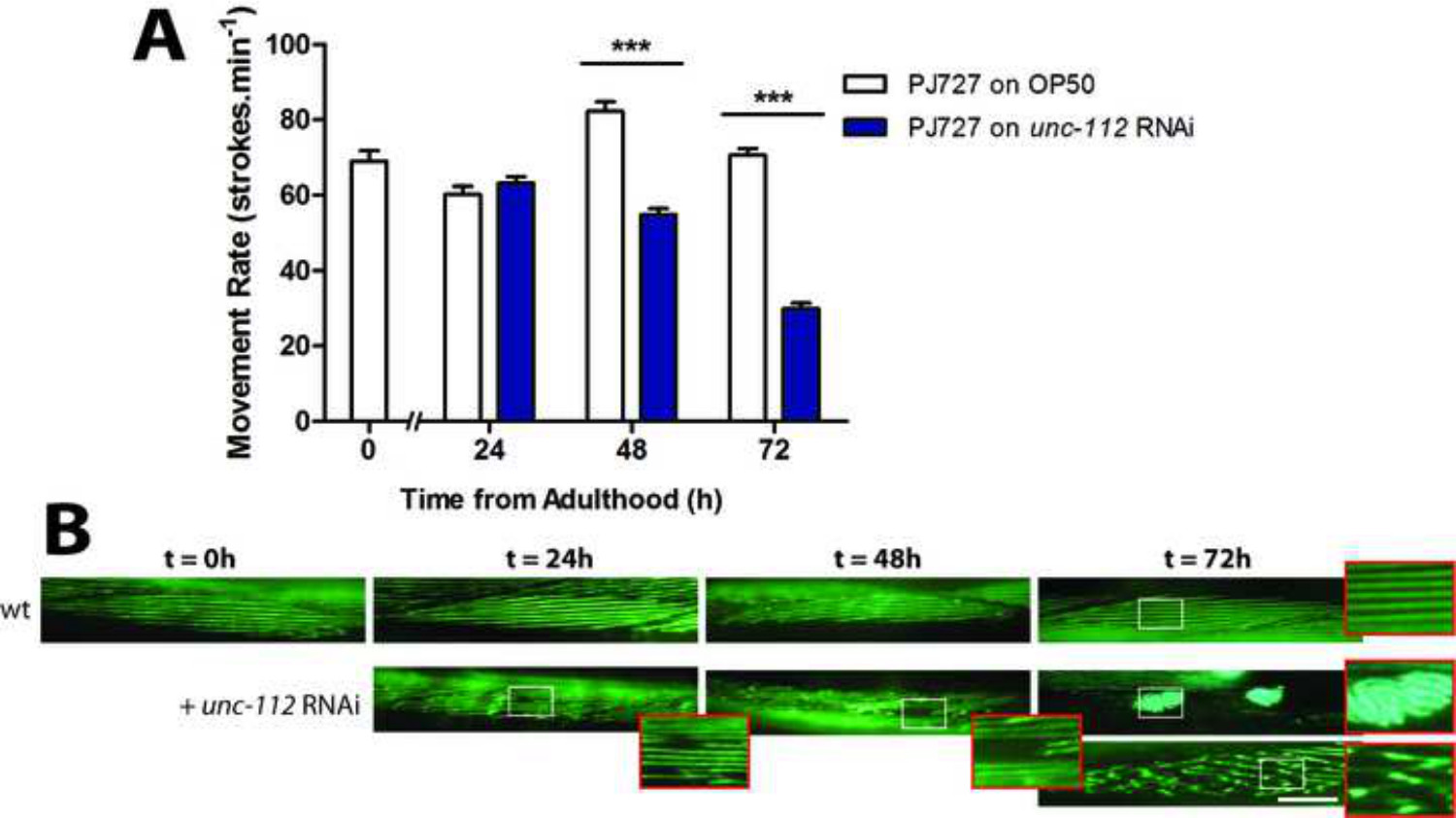
Figure 3: (A) unc-112 RNAi causes a significant movement defect at t = 72 hr. (B) Assessment of arrayed A-band structure within body wall muscle. In wt, A-bands in muscle appear as straight lines at young adulthood (t = 0 hr) and remain largely uniform until beyond 72 hr of adulthood (see wt zoom). unc-112 RNAi causes the sarcomeres to lose architecture in small areas of the muscle (t = 24 hr), collapsed and missing sarcomeres (t = 48 hr), aggregation (t = 72 hr top panel and zoom) and loss of linearity of sarcomeres (t = 72 hr bottom panel and zoom). Scale bars represent 25 µM and zooms are 2.5X.
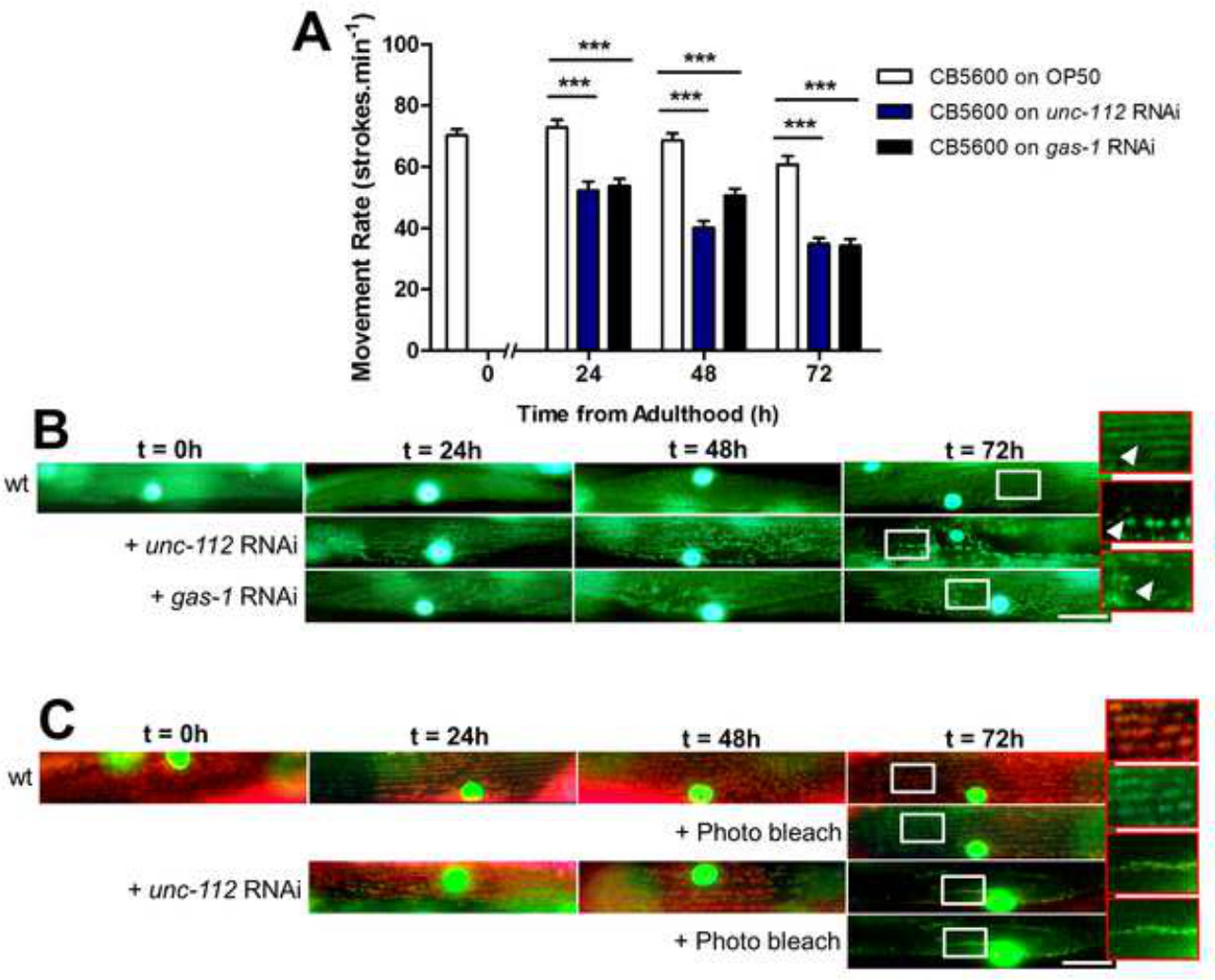
Figure 4: (A) unc-112 or gas-1 RNAi causes a movement defect. (B) Assessment of mitochondrial structure within body wall muscle. Mitochondria in muscle appear as networked straight lines at young adulthood (wt). Treatment of animals with unc-112 RNAi results in mitochondrial fragmentation as previously observed between 24 hr and 72 hr of adulthood (zooms and arrow)13. Likewise, RNAi against gas-1 results in fragmentation of the mitochondrial networks as previously observed26. These are present as small gaps in the mitochondrial network at t = 24 hr and progress to widespread disorganization at 72 hr (see zoom and arrow). (C) Assessment of mitochondrial membrane potential in vivo. In animals also containing GFP localized to mitochondria, C32H32Cl2N2O accumulates in body wall muscle mitochondria and appears orange under a triple-pass filter from young adulthood (t = 0 hr) until 72 hr post adulthood. Treatment with unc-112 RNAi causes a progressive loss of membrane potential as shown at 72 hr where the remaining mitochondria show less accumulation of C32H32Cl2N2O vs. wt. Photobleaching for 10 sec using 540/25 nm light bleaches the red from the MitoTracker and reveals the GFP localized to muscle mitochondria (+ Photo bleach). Scale bars represent 25 µm and zooms in are 2.5X.
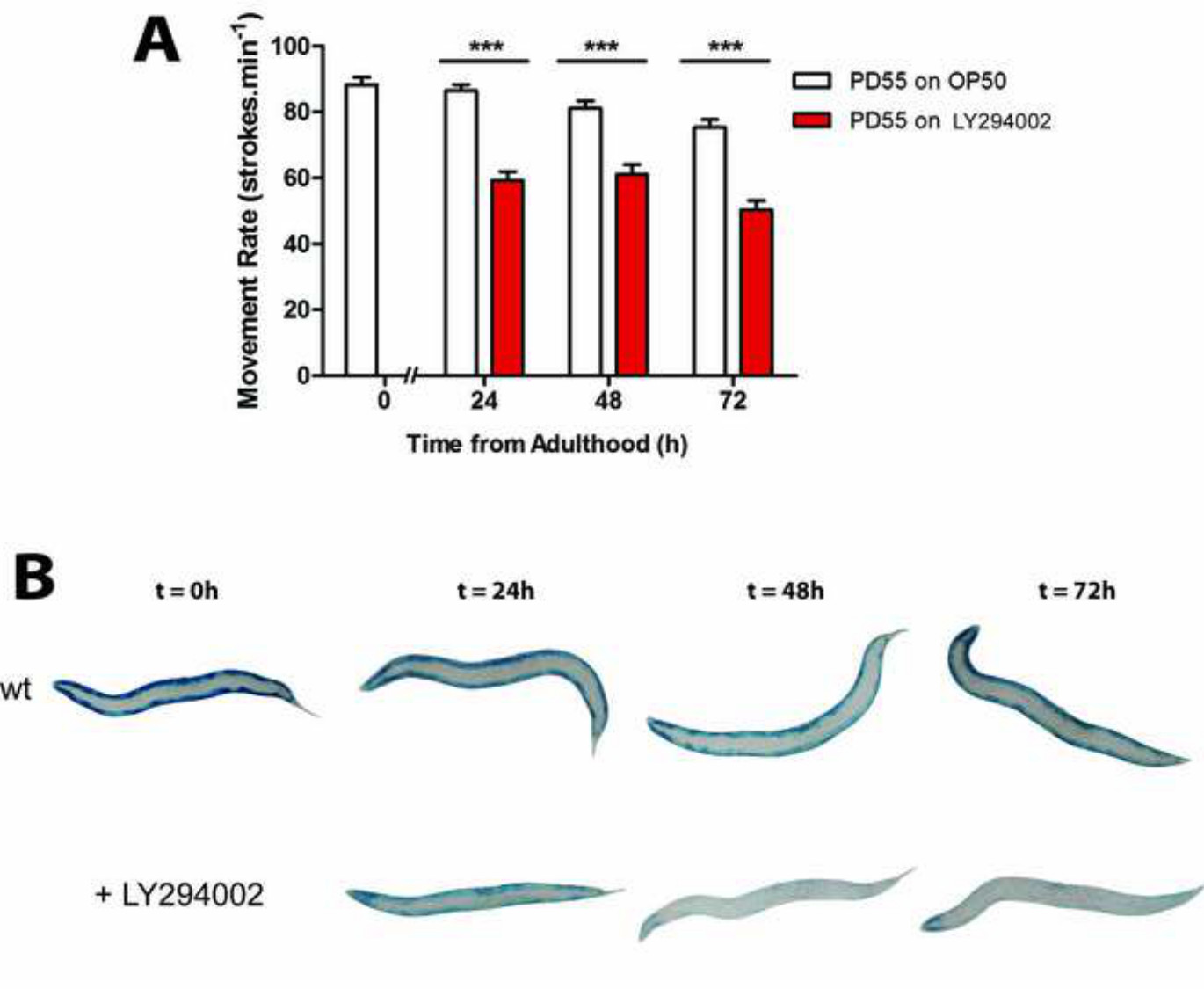
Figure 5: (A) Treatment with LY-294002 animals induces a movement defect. (B) Assessment of protein degradation using a β-galactosidase assay. Age synchronized wt L1 larvae are grown to young adulthood at 20 °C (t = 0 hr) before transferring to NGM seeded with OP50 (wt control) or NGM seeded with LY-294002 (PI3K inhibitor treatment) for 24 hr at 20 °C. In A, approximately 20–30 animals are stained for β-galactosidase activity (blue) at t = 0 hr and after 24 hr, 48 hr and 72 hr.
Discussion
These methods allow the exploration of muscle from the macrophysiology of movement to identifying subcellular defects that are sufficient to cause a movement defect. When combined these methods allow detailed analysis of muscle. The insight gained allows further testing of muscle-oriented hypotheses that pertain to general physiology, pathophysiology and ageing.
Movement Assays
A movement defect indicates a disruption of normal neuromuscular function. This may be specific to nerves or muscle or it may be common between several tissues. The most difficult stages of completing movement assays are selecting worms which are representative of the population and/or the treatment, and also ensuring that the experimenter does not injure the animal upon transfer to M9. It is important to have a large sample size to obtain a representative and accurate assessment of movement. When assaying highly penetrant effects, for example as frequently seen in mutants, a sample size of ten animals each assessed for movement ten times is sufficient to find statistically significant differences versus wild-type. However, the simplicity of movement assays and ease of culturing C. elegans mean that hundreds of worms can quickly be assessed in order to ensure quantitatively robust results. When assaying effects that appear present in only a portion of a population it is important to determine what proportion of the population appears to be affected as well as the severity of defect in the most affected animals. In such situations larger numbers of animals should be assessed in order to provide the minimal data for proportion of population affected. Frequently both drug and RNAi treatments will produce severe movement defects in a small proportion of the population. In some cases increasing the dose of the treatment and or the method of delivery will increase the proportion of affected animals and running dose response curves may be useful in determining the optimum concentration of a drug or RNAi. A second limitation of this movement assay is that the worms are manipulated to assess movement. Thus, the worms are both stimulated and subjected to some degree of osmotic stress when picked into liquid. The degree of osmotic stress can be limited by using M9 or BU buffer19 as opposed to water. Some of these limitations are alleviated by measuring habitual movement on plates using commercially available tracking systems34 or free plug ins for ImageJ35. Measuring the movement rate of an animal can provide important information regarding overall muscle health, including changes in function that can be measured throughout the lifespan and the non-invasiveness of this method is key for prospective life-long studies of muscle function.
Imaging of the Contractile Apparatus
The worm strain used here to image contractile apparatus structure was PJ72736 which expresses a myosin GFP fusion protein that is localized to sarcomeres within the body wall muscles. Use of this strain enables visualization of sarcomere structure in living animals. Similar to the movement assay described above, a key advantage of using GFP labeled sarcomeres to assess sarcomere structure is that the method is relatively non-invasive and therefore can be used to prospectively follow sarcomere structure in individual animals throughout the lifespan. The greatest difficulty with this method is that the worms that being imaged are still alive and therefore moving, which makes it difficult to get well-focused images. Several methods can be employed to reduce movement and make imaging simpler; these include anesthetizing or paralyzing the worms and immobilization via pressure, suction, or use of a high viscosity solution. Each of these methods of immobilization has the drawback that it can itself alter neuromuscular function. Therefore, it is essential to include appropriate untreated controls in the analysis. It may also be prudent to utilize at least two independent methods of immobilization to confirm results are not due to problems with the immobilization method, depending upon how widely used the immobilization method chosen is for the question under study. Here we have used simple pressure from the coverslip onto the worm to induce reduced movement. After placing the coverslip, the liquid beneath the coverslip will evaporate and the coverslip will consequently begin to produce more pressure upon the worms, typically this takes 2-5 min to produce enough reduced movement to enable easy imaging.
It is essential to monitor the worms closely after the coverslip has been introduced as the pressure will eventually increase enough to cause the worms to burst from excessive pressure, typically 10-15 min after coverslip addition. Additional buffer can be introduced with the aid of a pipet tip around the edges of the coverslip to avoid crush injury to the worms. Nail varnish can be used to seal the edges of the cover slip and prevent evaporation, although this prevents the retrieval of animals from under the cover slip, if desired. Similarly, the use of silicone beads in the solution can help reduce the amount of pressure the coverslip places on the worms.
Just as with assessing for a movement defect, it is important to consider the penetrance of effect. Penetrance may be considered high if 80-100% animals display a phenotype on the NGM plate, partial if 21-79% and low if ≤20% of the population display an affect. For highly penetrant effects, smaller sample sizes can be used to produce significant quantitative data on sarcomere defects. In cases where the effect has a low penetrance, selection of animals that display a clear movement defect in response to drug or RNAi treatment may be useful in assaying sarcomere defects in animals most affected by the treatment. However, it is not necessarily the case that the extent of treatment effect on movement and subcellular structure is the same. Thus, it may be desirable to examine the extent of defects present in a large population, for example 100 animals. This enables understanding of the distribution of defects throughout a population. It may also be desirable to examine the extent of the defect in the most severely affected animals and/or examine the correlation between extent of sarcomere defect and extent of movement defect. Potential difficulties aside, this method is quicker and easier than other methods of assessing sarcomere structure. This speed and ease is, however, subject to a key limitation, sarcomeres containing GFP fusion proteins are not entirely normal37 and therefore assessment of disrupted sarcomeres should be confirmed using additional more labor-intensive methods. These methods include use of polarized light38, phalloidin staining39, and indirect immunofluorescence40. Of these methods, only polarized light is relatively non-invasive; the other methods require fixation and therefore cannot be used in prospective studies of individual animals, rather they can only be used to confirm, cross sectionally, results from such studies.
Imaging of Mitochondrial Networks
The worm strain used for the mitochondrial imaging experiments was CB56007 which expresses a GFP fusion protein localized to the mitochondria and a GFP β-galactosidase fusion protein localized to the nuclei, both in the body wall muscles. As with use of PJ727 for imaging sarcomeres, use of this strain enables visualization of mitochondrial network structure in living animals. Thus, this strain can be used to assess dynamic changes that occur, for example reduced mitochondrial fusion and/or increased mitochondrial fission41. Also as for imaging sarcomeres, the greatest difficulty with this method is that the worms being imaged are still alive and moving, making it difficult to get well-focused images. While several methods, as discussed in greater detail above, can be employed to reduce movement, mitochondria appear particularly sensitive to interventions that induce reduced movement. For example, most commonly used anesthetics target components of the mitochondrial respiratory chain and are therefore must be used with caution in studies of normal mitochondrial structure and function. Similarly, most paralytic agents must be used with caution in studies of normal mitochondrial structure and function, as most paralytic agents cause less signal to pass through the neuromuscular junction and functional denervation of muscle is sufficient to induce mitochondrial fragmentation. The experiments in this study employed pressure to immobilize animals but others have successfully used levamisole42, although it is essential to image animals immediately.
Lastly, various bacterial contaminants in cultures, for example B. subtilis, can induce mitochondrial fragmentation; therefore it is essential to include appropriate untreated controls in the analysis. For highly penetrant effects, smaller sample sizes can be used to produce significant quantitative data on mitochondrial network defects. However, due to the prevalence of minor defects in the mitochondrial network, this small number of animals is likely to be twice the number needed for assessment of sarcomere structure. In cases where the effect has a low penetrance, selection of animals clearly displaying a movement defect in response to drug or RNAi treatment may be useful in assaying mitochondrial defects in animals most affected by the treatment. However, as mentioned above, it is not necessarily the case that the extent of treatment effect on movement and subcellular structure is the same. Thus, it may be desirable to examine the extent of defects present in a large population, for example 150-200 animals, as well as the extent of disruption in the most severely affected animals and the presence or absence of extent of disruption with extent of movement defect. Other strains with fluorescently labeled mitochondria can be used to confirm results obtained in CB5600, however, the use of different fluorescent proteins can influence the baseline networking of mitochondria. For example, the use of a TOMM-20 RFP fusion protein to visualize mitochondria appears to cause increased baseline mitochondrial fragmentation versus the use of mitochondrially localized GFP17. While other methods such as immunofluorescence and electron microscopy can be used to assess mitochondrial structure, they do not allow the in vivo assessment of mitochondrial dynamics because a fixation step is required. Other methods such as the use of mitochondrially localized dyes can be used without fixation and therefore can also be used in place of use of mitochondrially localized GFP. As discussed in the next section, use of such dyes also allows crude assessment of in vivo mitochondrial function.
Assessing Mitochondrial Membrane Potential In Vivo in C. elegans
A variety of dyes are available for labeling mitochondria28. These dyes provide an alternative method of visualizing mitochondrial network structure in live C. elegans. Many of these dyes also allow crude assessment of mitochondrial functionality in vivo because they accumulate in mitochondria based upon the mitochondrial membrane potential. Here we have used C32H32Cl2N2O which is ingested through the digestive tract, with some additionally entering the worm via the cuticle because of the permeabilization afforded by being dissolved in 0.5% DMSO. Following entry into the cell C32H32Cl2N2O is oxidized and sequestered by the mitochondria where it binds sulphur containing amino acids (e.g. methionine and cysteine). The formation of these dye-peptide complexes means C32H32Cl2N2O remains in the mitochondria once labeled28. A key difficulty with the use of mitochondrial dyes is that they will label all mitochondria in the animal. Therefore we have performed labeling in CB5600, the same strain described above for assessing muscle mitochondrial network structure. By using a red mitochondrial dye, a strain of worms expressing GFP in muscle mitochondria, and a triple pass filter, it is relatively straightforward to image only mitochondria in muscle by finding mitochondria that are orange by colocalization of red dye and GFP labeled mitochondria. The most difficult step in performing this colocalization approach is imaging because the dye is photo-bleached within seconds under high intensity fluorescent light. This effect can be limited by keeping the fluorescence on low power until the experimenter is ready to image and then adjusting the fluorescence intensity accordingly. Once one is experienced with the use of dyes another approach is to utilize confocal microscopy with Z-stacks to image only mitochondria in the right tissue; the mitochondrial networks in muscle are quite distinct compared to other tissues. Unfortunately, the inability of C32H32Cl2N2O to leave mitochondria28 means that, unlike the sarcomere and mitochondrial imaging techniques discussed above, it cannot be used to prospectively follow loss of mitochondrial function with time, unless C32H32Cl2N2O is added at discrete time points to separate animal populations. This limitation can be overcome by using dyes such as JC-10 that do leave the mitochondria upon collapse of membrane potential43. Mitochondria can also be isolated from C. elegans30 for subsequent biochemical analyses. Such extraction allows quantification of the mitochondrial membrane potential by quantifying the rate of lipophilic cationic dye uptake using flow cytometry or a fluorescent plate reader44. Having established an impaired mitochondrial membrane potential, it is also possible to determine if the loss of proton gradient translates into a loss of ATP production using a sensitive bioluminometric luciferase-based method45.
Assessing Protein Degradation in C. elegans
The worm strain used here for assessing muscle protein degradation was PD55, which contains a muscle specific β-galactosidase that is continuously synthesized throughout development until adulthood is reached and which remains stable in the cytosol for the next 72-96 hr19. Thus, measurement of β-galactosidase levels during development predominantly indicates the impact of interventions on synthesis from the myosin promoter whereas the loss of β-galactosidase during adulthood indicates an intervention has triggered increased cytosolic protein degradation19. The key step in assessing β-galactosidase activity is to ensure effective desiccation. Failure to effectively desiccate the animals results in poor provision of the X-gal substrate and therefore poor blue color in body-wall muscles of the animals. To ensure good desiccation the seal on the desiccator should be checked and the sample should be checked at 5-10 min intervals throughout desiccation to assess progress (i.e., the 20 µl sample should have dried within 10 min and the remaining 20 min is to ensure comprehensive desiccation). The β-galactosidase assay is an activity assay therefore an appropriate control is essential. Additionally, decreased staining of adults in a treatment condition relative to control does not prove that degradation is occurring; rather it indicates decreased enzymatic activity in response to the treatment. To confirm degradation, more labor-intensive western blot analysis must be completed against β-galactosidase13 and this allows quantification protein degradation in small populations of counted worms. Western blot band densities can be quantified using ImageJ46. Another limitation of this method is that the use of transgenic proteins does not inform as to physiologic targets of degradation and for such answers proteomic and/or targeted hypothesis driven western blots must be employed13. Lastly, as the synthesis of the transgenic protein employed in these studies shuts off in early adulthood19, other methods must be utilized when wishing to examine alterations in muscle protein synthesis in fully developed C. elegans muscle; for example, stable or radioactive isotope methods.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by a grant from the US National Institutes of Health National Institute for Arthritis and Musculoskeletal and Skin Diseases (AR-054342) to NJS. CJG was funded by a Doctoral training studentship funded by the University of Nottingham. JJB was funded by an MRC Doctoral training award (J500495). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Materials
| Name | Company | Catalog Number | Comments |
| MitoTracker Red CMXRos (C32H32Cl2N2O) | Invitrogen | M-7512 | Red mitochondrial dye which accumulates based on a negative membrane potential |
| DMSO | Thermo Scientific | 67-63-5 | |
| KH2PO4 | Sigma | 7778-77-0 | |
| Na2HPO4 | BDH | 301584L | |
| NaCl | Sigma-Aldrich | 7647-14-5 | |
| MgSO4 | Sigma | M-7506 | |
| Microscopy Slides | Starfrost | K220 | |
| Microscopy Cover Slips | VW2 International | 631-0124 | |
| 1.5 ml Eppendorph Tubes | Fisher Scientific | FB74031 | |
| Dessicator | Nalgene | D2672 | |
| Nikon Microscope | Nikon | H600L | Nikon H600L microscope with proprietary software |
| Nikon Camera | Nikon | DS-Fi1 | Nikon Digital Sight DS-Fi1 digital camera |
| Zeiss Microscope | Zeiss | Ax10 | Zeiss AX10 microscope with an Axiocam MRC digital camera and Axiovision LE software and a triple-pass filter |
| Plates 9 cm | Starstedt | 82-1473 | |
| Plates 6 cm | Gosselin | BP53-01 | |
| Potassium Ferrocyanide | Sigma-Aldrich | 14459-95-1 | |
| MgCl2 | Sigma-Aldrich | 7786-30-3 | |
| Na3PO4 | Sigma-Aldrich | 7601-54-9 | |
| 5-Bromo-4-chloro-3-indolyl β-D-galactopyranoside | Sigma-Aldrich | 7240-90-6 | |
| N,N-dimethylformamide | Sigma-Aldrich | 68-12-2 |
References
- Wannamethee, S. G., Shaper, A. G., Lennon, L., Whincup, P. H. Decreased muscle mass and increased central adiposity are independently related to mortality in older men. The American Journal of Clinical Nutrition. 86, 1339-1346 (2007).
- Marquis, K., et al. Midthigh Muscle Cross-Sectional Area Is a Better Predictor of Mortality than Body Mass Index in Patients with Chronic Obstructive Pulmonary Disease. American Journal of Respiratory and Critical Care Medicine. 166, 809-813 (2002).
- Metter, E. J., Talbot, L. a. u. r. a. A., Schrager, M., Conwit, R. Skeletal Muscle Strength as a Predictor of All-Cause Mortality in Healthy Men. Journal of Gerontology: Biological Sciences. 57, 359-365 (2002).
- Hairi, N. N., et al. Loss of Muscle Strength, Mass (Sarcopenia), and Quality (Specific Force) and Its Relationship with Functional Limitation and Physical Disability: The Concord Health and Ageing in Men Project. JAGS. 58, 2055-2062 (2010).
- FitzGerald, S. J., et al. Muscular Fitness and All-Cause Mortality: Prospective Observations. Journal of Physical Activity and Health. 1, 7-18 (2004).
- Consortium, C. e. S. Genome sequence of the nematode C. elegans: a platform for investigating biology. Science. 282, 2012-2018 (1998).
- Timmons, L., Fire, A. Specific interference by ingested dsRNA. Nature. 395, 854-854 (1998).
- Sulston, J. E., Horvitz, H. R. Post-embryonic cell lineages of the nematode. Caenorhabditis elegans Dev Biol. 56, 110-156 (1977).
- White, J., Wood, W. B. . The Anatomy. In The nematode C. elegans. , (1988).
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics. 77, 71-94 (1974).
- Moerman, D. G., Williams, B. D. Sarcomere assembly in C. elegans muscle. The C. elegans Research Community, WormBook. , (2006).
- Rual, J. F., et al. Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome Res. 14, 2162-2168 (2004).
- Etheridge, T., et al. Calpains Mediate Integrin Attachment Complex Maintenance of Adult Muscle in Caenorhabditis elegans. PLoS Genet. 8, 1002471 (2012).
- Shephard, F., Adenle, A. A., Jacobson, L. A., Szewczyk, N. J. Identification and Functional Clustering of Genes Regulating Muscle Protein Degradation from amongst the Known C. elegans Muscle Mutants. PLoS ONE. 6, e24686 (2011).
- Lehmann, S., Bass, J. J., Szewczyk, N. J. Knockdown of the C. elegans Kinome identifies Kinases required for normal protein Homeostasis, Mitochondrial network structure, and Sarcomere structure in muscle. Cell Communication & Signaling. 11, (2013).
- Herndon, L. A., et al. Stochastic and genetic factors influence tissue-specific decline in ageing. C. elegans. Nature. 419, 808-814 (2002).
- Munoz-Lobato, F., et al. Protective role of DNJ-27/ERdj5 in Caenorhabditis elegans models of human neurodegenerative diseases. Antioxid Redox Signal. 5, 5 (2013).
- Chalfie, M., Tu, Y., Euskirchen, G., Ward, W., Prasher, D. Green fluorescent protein as a marker for gene expression. Science. 263, 802-805 (1994).
- Zdinak, L. A., et al. Transgene-coded chimeric proteins as reporters of intracellular proteolysis: starvation-induced catabolism of a lacZ fusion protein in muscle cells of Caenorhabditis elegans. J Cell Biochem. 67, 143-153 (1997).
- Berkowitz, L. A., Knight, A. L., Caldwell, G. A., Caldwell, K. A. Generation of Stable Transgenic C. elegans Using Microinjection. J Vis Exp. 18, e833 (2008).
- Szewczyk, N. J., Peterson, B. K., Barmada, S. J., Parkinson, L. P., Jacobson, L. A. Opposed growth factor signals control protein degradation in muscles of Caenorhabditis elegans. The EMBO Journal. 26, 935-943 (2007).
- Stiernagle, T. . The C. elegans Research Community, WormBook. , 1551-8507 (2006).
- Gaud, A., et al. Prednisone reduces muscle degeneration in dystrophin-deficient Caenorhabditis elegans. Neuromuscular Disorders. 14, 365-370 (2004).
- Dillin, A., et al. Rates of Behavior and Aging Specified by Mitochondrial Function During Development. Science. 298, 2398-2401 (2002).
- Bakeeva, L., Chentsov, Y., Skulachev, V. Mitochondrial framework (reticulum mitochondriale) in rat diaphragm muscle. Biochimica et Biophysica Acta. 501, 349-369 (1978).
- Ichishita, R., et al. An RNAi screen for mitochondrial proteins required to maintain the morphology of the organelle in Caenorhabditis elegans. J Biochem. 143, 449-454 (2008).
- Mitchell, P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 191, 144-148 (1961).
- Chazotte, B. Labeling mitochondria with MitoTracker dyes. Cold Spring Harb Protoc. 1, 990-992 (2011).
- Seo, A. Y., et al. New insights into the role of mitochondria in aging: mitochondrial dynamics and more. J Cell Sci. 123, 2533-2542 (2010).
- Brys, K., Castelein, N., Matthijssens, F., Vanfleteren, J. R., Braeckman, B. P. Disruption of insulin signalling preserves bioenergetic competence of mitochondria in ageing Caenorhabditis elegans. BMC Biol. 8, 1741-7007 (2010).
- Dorrens, J., Rennie, M. J. Effects of ageing and human whole body and muscle protein turnover. Scandinavian Journal of Medicine & Science in Sports. 13, 26-33 (2003).
- Rennie, M. J., et al. Depressed protein synthesis is the dominant characteristic of muscle wasting and cachexia. Clinical Physiology. 3, 387-398 (1983).
- Mitch, W. E., Goldberg, A. L. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Engl J Med. 335, 1897-1905 (1996).
- Husson, S. J., Costa, W. S., Schmitt, C. . The C. elegans Research Community, WormBook. , 1551-8507 (2012).
- Kawano, T., et al. An imbalancing act: gap junctions reduce the backward motor circuit activity to bias C. elegans for forward locomotion. Neuron. 72, 572-586 (2011).
- Fostel, J. L., Benner Coste, L., Jacobson, L. A. Degradation of transgene-coded and endogenous proteins in the muscles of Caenorhabditis elegans. Biochemical and Biophysical Research Communications. 312, 173-177 (2003).
- Meissner, B., et al. An integrated strategy to study muscle development and myofilament structure in Caenorhabditis elegans. PLoS Genet. 5, (2009).
- Rogalski, T. M., Gilbert, M. M., Devenport, D., Norman, K. R., Moerman, D. G. DIM-1, a novel immunoglobulin superfamily protein in Caenorhabditis elegans, is necessary for maintaining bodywall muscle integrity. Genetics. 163, 905-915 (2003).
- Gieseler, K., Grisoni, K., Segalat, L. Genetic suppression of phenotypes arising from mutations in dystrophin-related genes in Caenorhabditis elegans. Curr Biol. 10, 1092-1097 (2000).
- Wilson, K. J., Qadota, H., Benian, G. M. Immunofluorescent localization of proteins in Caenorhabditis elegans muscle. Methods Mol Biol. 798, 171-181 (2012).
- Chan, D. C. Mitochondrial Fusion and Fission in Mammals. Annual Review of Cell and Developmental Biology. 22, 79-99 (2006).
- Ray, A., Martinez, B. A., Berkowitz, L. A., Caldwell, G. A., Caldwell, K. A. Mitochondrial dysfunction, oxidative stress, and neurodegeneration elicited by a bacterial metabolite in a C. elegans Parkinson's model. Cell Death Dis. 9, 513 (2014).
- Perry, S. W., Norman, J. P., Barbieri, J., Brown, E. B., Gelbard, H. A. Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. Biotechniques. 50, 98-115 (2011).
- Scaduto, R. C., Grotyohann, L. W. Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys J. 76, 469-477 (1999).
- Wibom, R., Hagenfeldt, L., von Döbeln, U. Measurement of ATP production and respiratory chain enzyme activities in mitochondria isolated from small muscle biopsy samples. Analytical Biochemistry. 311, 139-151 (2002).
- Gassmann, M., Grenacher, B., Rohde, B., Vogel, J. Quantifying Western blots: Pitfalls of densitometry. Electrophoresis. 30, 1845-1855 (2009).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved