A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Blue-hazard-free Candlelight OLED
In This Article
Summary
We present a protocol for the fabrication of a blue-hazard-free candlelight organic light emitting diode (OLED) for eye protection and melatonin secretion.
Abstract
A candlelight-style organic light emitting diode (OLED) is a human-friendly type of lighting because it is blue-hazard-free and has a low correlated color temperature (CCT) illumination. The low CCT lighting is deprived of high-energy blue radiation, and it can be used for a longer duration before causing retinal damage. This work presents the comprehensive protocols for the fabrication of blue-hazard-free candlelight OLEDs. The emission spectrum of the OLED was characterized by the maximum exposure time limit of the retina and the melatonin suppression sensitivity. The devices can be fabricated using dry and wet processes. The dry-processed OLED resulted in a CCT of 1,940 K and exhibited a maximum retinal exposure limit of 1,287 s at a brightness of 500 lx. It showed 2.61% melatonin suppression sensitivity relative to 480 nm blue light. The wet-processed OLED, where the spin coating is used to deposit hole injection, hole transport, and emissive layers, making fabrication fast and economical, produced a CCT of 1,922 K and showed a maximum retinal exposure limit of 7,092 at a brightness of 500 lx. The achieved relative melatonin suppression sensitivity of 1.05% is 86% and 96% less than that of the light emitting diode (LED) and compact fluorescent lamp (CFL), respectively. Wet-processed blue-hazard-free candlelight OLED exhibited a power efficiency of 30 lm/W, which is 2 times that of the incandescent bulb and 300 times that of the candle.
Introduction
Nowadays, lighting sources like LED and CFL are abundantly used for indoor and outdoor illumination, partly for energy-saving reasons. However, these lights are rich in blue emission, showing a higher tendency to cause blue-hazards. LED and CFL emit a spectrum enriched with blue light, leading to irreversible damage to retinal cells1,2,3,4. Blue light or intense white light with high CCT suppresses the secretion of melatonin, an oncostatic hormone, which may disrupt the circadian rhythm5,6 and sleeping behavior7,8. Melatonin, an essential hormone for the circadian rhythm, is synthesized in the pineal gland9. A high level of melatonin is observed during the dark period during the 24-h light-dark cycle10. However, intensive light at night suppresses its synthesis and disrupts the circadian rhythm11. Melatonin suppression due to overexposure to bright lights at night can be a risk factor for breast cancer in women12,13,14. Besides these hazards, blue light interrupts the activities of nocturnal amphibians and can be threatening to ecological protection. It has also been reported that LED lighting in museums is discoloring the actual colors of oil paintings painted by Van Gogh and Cézanne15,16.
Thus, a blue-emission free and low CCT candle-like organic LED (OLED) can be a good substitute for LED and CFL. Candles emit a blue-hazard-free and low CCT (1,914 K) illumination, as well as a high-quality (high color rendering index, CRI) emission spectrum. However, most of the electricity-driven lighting devices emit intense blue light with a comparatively high CCT. For example, the lowest CCT is about 2,300 K for incandescent bulbs, while it is 3,000 or 5,000 K for warm or cold white fluorescent tubes and LED luminaires. So far, low CCT OLEDs nearly free of the blue emission have been fabricated for human-friendly lighting. In 2012, Jou's group reported a physiologically friendly, dry-processed, single emissive layer OLED with a CCT of 1,773 K and a power efficiency of 11.9 lm/W17. The device exhibited a much lower CCT as compared to the incandescent bulb (2,300 K), while its power efficiency was not acceptable from an energy-saving point of view. They reported another dry-processed candlelight-style OLED by using double emissive layers along with a carrier modulation layer18. It exhibited a low CCT of 1,970 K and a power efficiency of 24 lm/W. Later on, a dry-processed OLED consisting of three emissive layers along with a carrier modulation layer was reported19. Its power efficiency was from 21 to 3 lm/W and varied with the CCT, which ranged from 2,500 K to 1,900 K. In 2014, Hu et al. reported a dry-processed hybrid OLED with double emissive layers separated by an interlayer, which showed a high power efficiency of 54.6 lm/W and a low CCT of 1,910 K20. Recently, Jou's group has fabricated a high-efficiency candlelight-style OLED by employing double emissive layers21. It exhibited a high power efficiency of 85.4 lm/W with a CCT of 2,279 K. Until now, all efforts have been made to develop high efficiency, low CCT candlelight-style OLED devices by utilizing dry processes and complicated device architectures17,18,19,20,21,22. Devising a candlelight OLED with wet-process feasibility while simultaneously having a low CCT, a high power efficiency, and a high light quality is a challenge. No study has been developed to describe the emission spectrum sensitivity of a given light source with respect to the blue light. The quality of light at night can be decided/improved to minimize the suppression of melatonin secretion.
There are some reported models that calculate the amount of suppression. Firstly, Brainard et al.23 and Thapan et al.24 reported the spectral sensitivity by using monochromatic light. Later on, the effect of polychromatic light on melatonin suppression was described25,26. The latter is adopted in this study, since most of the commercially available luminaires or novel lighting sources are polychromatic and span over the entire visible range (i.e., from deep red to violet).
In this work, we present comprehensive protocols for the fabrication of blue-hazard-free candlelight OLEDs via dry and wet processes. In both processes, the device architecture is simplified by employing a single emissive layer without any carrier modulation layers. The electroluminescent (EL) spectrum of the fabricated OLED is analyzed for the retinal exposure limit and for the level of melatonin secretion suppression. A maximum exposure limit of emitted light to the retina is calculated by using the theoretical aspect that was reported by the International Electrotechnical Commission (IEC) 62471 standard27,28. The maximum exposure limit "t" is calculated by using the emission spectrum of each OLED at the brightness of 100 and 500 lx, sufficient for home and office illumination, respectively. All related calculation steps are sequentially given in the protocol section. Further, the effect of lighting on the melatonin suppression sensitivity is calculated by following the equations of the action spectrum of melatonin suppression29. The calculation is done by following the steps given in the protocol section. The calculated values of the maximum exposure limit "t" and the melatonin suppression sensitivity (%) with respect to CCT are given in Table 3.
Protocol
NOTE: All the materials used are non-carcinogenic, non-flammable, and non-toxic.
1. Fabrication of Blue-hazard-free Candlelight OLED
- Dry process
- Take a glass slide as a substrate to be coated with a 125 nm indium tin oxide (ITO) anode layer. Wash the substrate with 200 mL (50 mL of liquid detergent and 150 mL of deionized water) of soap solution. Rinse the substrate with deionized water. Dry the substrate with a nitrogen jet spray.
- Put the substrate on a glass slide holder and dip the slide holder in acetone solution in a beaker. Put the beaker in an ultrasonic bath. Sonicate the substrate at 50 °C for 10 min.
- Transfer the slide holder with the substrate to isopropanol solution in a beaker and again sonicate at 60 °C for 10 min.
- Take out the substrate from the beaker and put it in the UV/ozone slot for 10 min to dry. Clean the surface completely.
- Break the vacuum of the thermal evaporator chamber by closing the valve of the high vacuum and opening the valve of nitrogen gas to the chamber.
- Load the cleaned substrate in the chamber on the rotating substrate holder. For each layer that will be deposited, load 100 mg of each required organic material, 3 mg of lithium fluoride (LiF), and a 224 mg aluminum (Al) ingot into the crucible inside the chamber.
- Close the door of the chamber and wait for a high vacuum of 5×10-6 Torr. Once the high vacuum has been reached inside the chamber, start the deposition of the organic layers onto the substrate with ITO.
- Deposit a 5 nm hole injection layer at a deposition rate of 0.8-1 Å/s.
- Deposit a 25 nm transport layer at a deposition rate of 1-1.5 Å/s.
- Deposit a 30 nm emissive layer (8 wt.% green dye and 0.85 wt.% deep-red dye doped in 20 mg of a specified host) at a deposition rate of 1-1.5 Å/s.
- Deposit a 30 nm electron transport layer at a deposition rate of 1-1.5 Å/s.
- Deposit a 20 nm layer of electron transport co-evaporate with electron injection material at a deposition rate of 1-1.5 Å/s.
- Deposit a 1-nm electron injection layer of LiF at a deposition rate of 0.3-0.4 Å/s.
- Deposit a 100-nm cathode layer of Al at a deposition rate of 10-15 Å/s.
- Turn off the current controller and wait 10 min under high vacuum. Close the valve for high vacuum and open the valve for nitrogen gas to the chamber to break the high vacuum.
- Move the fabricated OLED device from the chamber to the atmosphere, and then transfer it to a glove box with an encapsulation machine under a nitrogen atmosphere.
- Encapsulate the fabricated OLED device with a top cover made of glass by using glue and then dry the glue by putting the device in UV radiation box for 110 s.
- Eject out the encapsulated OLED device from the glove box and transfer it to the darkroom for measurements.
- Wet process
- Clean the ITO-coated substrate by using the aforementioned cleaning procedures from steps 1.1.2 to 1.1.4.
- Take an aqueous solution of PEDOT: PSS (stored at 4 °C) to deposit the hole injection layer. Filter the solution in a vial by using a 25-mm diameter filter consisting of a nylon fabric with a pore size of 0.45 µm.
- In a vial, prepare the hole transport layer solution of 3,6-bis(4-vinylphenyl)-9-ethylcarbazole (VPEC)30 dissolved in chlorobenzene solvent in the ratio of 3 mg:1,000 µL. Sonicate the solution for 30 min in the ultrasonic bath and filter the sonicated solution in a vial with a 15-mm diameter filter consisting of a nylon fabric with a pore size of 0.45 µm.
- Prepare a solution for the emissive layer.
- Take 5 mg of the specified host material and dissolve it in tetrahydrofuran (THF) in a ratio of 10 mg:1,000 µL. Sonicate the host-solution at 50 °C for 30 min.
- Take 1 mg of each of the required guest materials and dissolve them in THF in a ratio of 1 mg:1,000 µL. Sonicate the guest-solution at 50 °C for 30 min.
- Filter each solution separately in vials with a 15 mm diameter filter consisting of a nylon fabric with a pore size of 0.45 µm.
- Mix the guest-solution into the host-solution according to the given weight percent (3 wt.% of yellow dye, 6 wt.% of orange-dye, and 12.5 wt.% of green dye), doping for the emissive layer.
- Transfer the vials of PEDOT: PSS, VPEC, and emissive layer solutions along with pre-cleaned substrate and pipette them into the glove box.
- Start coating the layers onto the substrate with ITO in the following sequence under a nitrogen atmosphere: the hole injection layer, hole transport layer, and emissive layer.
- Deposit a 35 nm hole injection layer by spin-coating a 750 µL solution of PEDOT: PSS at 4,000 revolutions per min (rpm) for 20 s.
- Dry the PEDOT: PSS layer at 120 °C for 40 min to remove residual solvent.
- Deposit a 10-nm hole transport layer by spin-coating a 400 µL solution of VPEC at 3,000 rpm for 20 s.
- Bake the layer at 120 °C for 20 min to remove residual solvent.
- Heat the layer at 230 °C for 40 min for a crosslinking reaction to occur before depositing the emissive layer30.
- Deposit a 20 nm emissive layer by spin-coating a 400-µL solution at 2,500 rpm for 20 min.
- Eject out the spin-coated substrate from the glove box to the atmosphere and transfer it to the thermal evaporator chamber for the further deposition of layers. Break the vacuum of the thermal evaporator chamber by closing the valve of the high vacuum and open the valve of the nitrogen gas to the chamber.
- Load the substrate in the chamber on the rotating substrate holder. Load the 45 mg of TPBi, 3 mg of LiF, and a 224 mg Al ingot into the crucible inside the chamber for the layers that will be deposited. Deposit the layers onto the substrate with the emissive layer in the following sequence.
- Deposit a 32 nm electron transport layer of TPBi at a deposition rate of 1-1.5 Å/s.
- Deposit a 1 nm electron injection layer of LiF at a deposition rate of 0.3-0.4 Å/s.
- Deposit a 100-nm cathode layer of Al at a deposition rate of 10-15 Å/s.
- Turn off the current controller and wait 10 min under the high vacuum. Follow the aforementioned procedures from steps 1.1.8 to 1.1.11 to complete the encapsulated OLED device.
- Calculation of the retina-permissible exposure limit "t":
- Measure the EL spectrum of the lighting device by using a spectroradiometer. The resulting EL spectrum is shown in Figure 1a.
- Measure the EL spectrum data (intensity versus wavelength) at a CCT.
- Convert the EL spectrum data to spectral radiance Eλ (normalized intensity versus wavelength). Change the spectrum to the format shown in Figure 1b.
- Use the spectral data from the blue light-weighted function for measuring the retinal hazard from lighting source (i.e., draw the blue light hazard function B(λ) with respect to the wavelength)28. The resulting plot is shown in Figure 1c.
- Calculate the value of the radiance (EB) of a given light source by using the spectral radiance Eλ and blue-hazard function B(λ) corresponding to each wavelength.
- Put the values of Eλ and B(λ) from the abovementioned plots into the following formula:
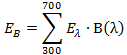 .....(1)
.....(1) - Get the numerical value of EB in W m-2.
- Put the value of EB in the maximum permissible retinal exposure limit "t" formula:
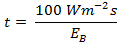 .....(2)
.....(2) - Acquire the exposure limit "t" with respect to the CCT of a given light source.
- Calculation for melatonin suppression sensitivity:
- Measure the EL spectrum of a given lighting device by using spectroradiometer. The resulting spectrum is shown in Figure 2a.
- Get the melatonin suppression power per quantum, SPQ, from the programmed data29. For a given monochromatic light λ, express the SPQ as follows:
SPQ (λ) = 10 (λr-λ)/C …………. (3)
The values of SPQ (λ) with respect to wavelength are given in Table 1, and the respective graph is shown in Figure 2b. - Use the photopic luminosity function V (λ) to convert SPQ (λ) into the melatonin suppression power per lux, SLC (λ), in order to give it a practical meaning. The values of V (λ) with respect to the wavelength are given in Table 2, and the respective graph is shown in Figure 2c.
- Express the correlated melatonin suppression power, SLC (λ), for a polychromatic light, as follows:29
SLC (λ) = ∫λSPQ(λ)SI(λ)dλ/∫ V(λ)SI(λ)dλ …………….. (4) - Put the values of the intensity SI (λ) from the EL spectrum of a given light source along with the values of SPQ (λ) and V(λ) with respect to the wavelength in the above formula and calculate the SLC (λ) as follows:
SLC (λ) =
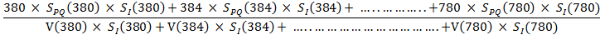
- Retrieve a numerical value of SLC (λ) in lx-1 from the above calculation. For example, by putting the SI (λ) from the EL spectrum of the given candlelight OLED with a CCT of 1,940 K, the melatonin suppression power is:
SLC (λ) = 90 lx-1 - Choose a reference light to calculate the relative melatonin suppression sensitivity of a given light source. The reference light can be a wavelength of 460 or 480 nm. Here, we choose a blue light of 480 nm as the reference light.
- Calculate the SLC (λ) for the reference blue light (480 nm) by using the abovementioned formula.
SLC (480 nm) = 3,445 lx-1 - Divide the SLC (λ) of a given light source by the SLC (480 nm) and multiply the quotient by 100 to get the melatonin suppression sensitivity percentage (%) of a given light relative to the reference blue light.
Relative melatonin suppression sensitivity =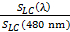 ×100 % ……….... (5)
×100 % ……….... (5)
NOTE: For example, relative melatonin suppression sensitivity =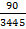 ×100% = 2.61%. Thus, the given candlelight OLED shows a melatonin suppression sensitivity of 2.61% relative to that of the 480-nm blue light.
×100% = 2.61%. Thus, the given candlelight OLED shows a melatonin suppression sensitivity of 2.61% relative to that of the 480-nm blue light.
Results
The current-voltage-luminance characteristics of the resulting candlelight OLEDs are measured by using an electrometer together with a 100 A luminance meter. The emission areas are 9 mm2 for all of the resulting dry-processed devices and are 25 mm2 for wet-processed devices. Here, we used a 125 nm ITO-coated glass substrate with a sheet resistance of 15 Ω/sq as an anode. It has a transparency greater than 84% (Table 4). All the OLED devices cons...
Discussion
The most critical steps in the fabrication of OLED devices are: 1) cleaning the glass substrate, 2) selecting the appropriate solvent, 3) dissolving the organic materials, 4) uniformly forming the film via spin-coating in the wet process, and 5) controlling the deposition rate and thickness of the organic layer during the thermal evaporation. Initially, cleaning the ITO anode coated substrate is a crucial step to achieve high efficiency. The glass substrate is cleaned with soap solution to remove greasy spots or layers. ...
Disclosures
We have nothing to disclose.
Acknowledgements
The authors would like to acknowledge the support in part from the Ministry of Economic Affairs and the Ministry of Science and Technology, Taiwan, via Grants MEA 104-EC-17-A-07-S3-012, MOST 104-2119-M-007-012, and MOST 103-2923-E-007-003-MY3.
Materials
| Name | Company | Catalog Number | Comments |
| ITO glass | Lumtech | 84% transparency | |
| poly(3,4-ethylenedioxythiophene) - poly(styrenesulfonate) (PEDOT/PSS) | UniRegion Bio-Tech | Stored at 4 °C, HOMO (eV) = -4.9, LUMO (eV) = -3.3 | |
| 4,4,4-tris(N-carbazolyl)triphenylamine (TCTA) | E-Ray Optoelectronics Technology co., Ltd | Non-toxic, HOMO (eV) = -5.7, LUMO (eV) = -2.3 | |
| tris(2-phenyl-pyridine) (Ir(ppy)3) | E-Ray Optoelectronics Technology co., Ltd | Non-toxic, HOMO (eV) = -5.6, LUMO (eV) = -3.9 | |
| 1,3,5-tris(N-phenylbenzimidazol-2-yl)benzene (TPBi) | Luminescence Technology corp. | Non-toxic, HOMO (eV) = -6.2, LUMO (eV) = -2.7 | |
| iridium(III) bis(4-phenylthieno[3,2-c]pyridinato-N,C 2’)acetylacetonate (PO-01) | Luminescence Technology corp. | Non-toxic, HOMO (eV) = -5.1, LUMO (eV) = -2.7 | |
| tris(2-phenylquinoline)iridium(III) (Ir(2-phq)3) | E-Ray Optoelectronics | Non-toxic, HOMO (eV) = -5.1, LUMO (eV) = -2.8 | |
| LiF | Echo chemicals | 99.98% | |
| Aluminium ingot (Al) | Guv team International pvt. ltd | 100.00% | |
| Acetone | Echo chemicals | 99.90% | |
| 2-Propanol | Echo chemicals | 99.90% | |
| Hole-injection material, WHI-001 | WAN HSIANG precision machinery co., Ltd | non-toxic, HOMO (eV) = -9.8, LUMO (eV) = -5.6 | |
| Hole-transport material, WHI-215 | WAN HSIANG precision machinery co., Ltd | non-toxic, HOMO (eV) = -5.4, LUMO (eV) = -2.5 | |
| host material, WPH-401 | WAN HSIANG precision machinery co., Ltd | non-toxic, HOMO (eV) = -5.8, LUMO (eV) = -2.7 | |
| Electron-injection material, WIT-651 | WAN HSIANG precision machinery co., Ltd | non-toxic, HOMO (eV) = -5.8, LUMO (eV) = -3.1 | |
| Electron-transpot material, WET-603 | WAN HSIANG precision machinery co., Ltd | non-toxic, HOMO (eV) = -5.9, LUMO (eV) = -2.6 | |
| Green dye, WPGD-832 | WAN HSIANG precision machinery co., Ltd | non-toxic, HOMO (eV) = -5.8, LUMO (eV) = -3.1 | |
| Deep-red dye, PER 53 | E-Ray Optoelectronics Technology co., Ltd | non toxic, HOMO (eV) = -5.1, LUMO (eV) = -2.4 |
References
- Melton, R. Ultraviolet and blue light. Rev opt. 2, 151 (2014).
- Singerman, L. J., Miller, D. G. Pharmacological Treatments for AMD. Rev Ophthalmol. 10, 88-90 (2003).
- . . International Energy Agency final report on potential health issues on SSL. , (2014).
- Pauley, S. M. Lighting for the human circadian clock: Recent research indicates that lighting has become a public health issue. Med. Hypotheses. 63, 588-596 (2004).
- Mills, P. R., Tomkins, S. C., Schlangen, L. J. M. The effect of high correlated colour temperature office lighting on employee wellbeing and work performance. J. Circadian Rhythm. 5, 1-9 (2007).
- Sato, M., Sakaguchi, T., Morita, T. The effects of exposure in the morning to light of different color temperatures on the behavior of core temperature and melatonin secretion in humans. Biol. Rhythm. Res. 36, 287-292 (2005).
- Arendt, J. Melatonin, circadian rhythms, and sleep. New Engl. J. Med. 343 (15), 1114-1116 (2000).
- Wiechmann, A. F. Melatonin: parallels in pineal gland and retina. Exp Eye Res. 42 (6), 507-527 (1986).
- Brown, G. M. Light, melatonin, sleep-wake cycle. J. pshychiatry. Neurosci. 19 (5), 345-356 (1994).
- Lewy, A. J., Wehr, T. A., Goodwin, F. K., Newsome, D. A., Markey, S. P. Light suppresses melatonin secretion in humans. Science. 210 (4475), 1267-1269 (1980).
- Stevens, R. G., Brainard, G. C., Blask, D. E., Lockley, S. W., Motta, M. E. Breast cancer and circadian disruption from electric lighting in the modern world. CA Cancer J. Clin. 64 (3), 207-218 (2014).
- Davis, S., Mirick, D. K., Stevens, R. G. Night-shift work, light at night, and risk of breast cancer. J. Natl. Cancer Inst. 93, 1557-1562 (2001).
- Kloog, I., Haim, A., Stevens, R. G., Barchanade, M., Portnov, B. A. Light at Night Co Distributes with Incident Breast but Not Lung Cancer in the Female Population of Israel. Chronobiology Intl. 25, 65-81 (2008).
- Monico, L. . S. Anal. Chem. 85 (2), 851-859 (2013).
- Jou, J. H. Organic light-emitting diode-based plausibly physiologically-friendly low color-temperature night light. Org. Electron. 13 (8), 1349-1355 (2012).
- Jou, J. H. Candlelight-style organic light-emitting diodes. Adv. Funct. Mater. 23 (21), 2750-2757 (2013).
- Jou, J. H. OLEDs with chromaticity tunable between dusk-hue and candle-light. Org. Electron. 14 (1), 47-54 (2013).
- Hu, Y., Zhang, T., Chen, J., Ma, D., Cheng, C. H. Hybrid organic light-emitting diodes with low color temperature and high efficiency for physiologically-friendly night illumination. Isr. J. Chem. 54, 979-985 (2014).
- Jou, J. H. Enabling a blue-hazard free general lighting based on candlelight-style OLED. Optics Express. 23 (11), A576-A581 (2015).
- Jou, J. H. High efficiency low color-temperature organic light emitting diodes with a blend interlayer. J. Mater. Chem. 21, 17850-17854 (2011).
- Brainard, G. G. Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. J Neurosci. 21 (16), 6405-6412 (2001).
- Thapan, K., Arendt, J., Skene, D. J. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 535 (Pt 1), 261-267 (2001).
- Bullough, J. D., Bierman, A., Figueiro, M. G., Rea, M. S. Letter On Melatonin Suppression from Polychromatic and Narrowband Light Lighting Research. Chronobiol. Int. 25 (4), 653-656 (2008).
- Rea, M. S., Figueiro, M. G., Bullough, J. D., Bierman, A. A model of phototransduction by the human circadian system. Brain Res Brain Res Rev. 50, 213-228 (2005).
- International Electrotechnical Commission. Photobiological safety of lamps and lamp systems. IEC 62471: 2006. , (2006).
- ICNIRP. ICNIRP guidelines on limits of exposure to incoherent visible and infrared radiation. Health Physics. 105 (1), (2013).
- Jou, J. H. Melatonin suppression extent measuring device. Patent. , (2012).
- Jou, J. H. Enabling high-efficiency organic light-emitting diodes with a cross-linkable electron confining hole transporting material. Org. Electron. 24, 254-262 (2015).
- Commission International de l’Éclairage. . Method of measuring and specifying colour rendering of light sources. , 16 (1995).
- Jou, J. H. A universal, easy-to-apply light-quality index based on natural light spectrum resemblance. Appl. Phys. Lett. 104, 203304-203309 (2014).
- Jou, J. H. Pseudo-natural light for displays and lighting. Adv. Optical mater. 3, 95-102 (2015).
- Jou, J. H. Wetprocess feasible candlelight OLED. J. Mater. Cem. C. , (2016).
- Kim, B. S. UV-ozone surface treatment of indium-tin-oxide in organic light emitting diodes. J. Korean Phys. Soc. 50, 1858-1861 (2007).
- Lee, T. W. Characteristics of solution-processed small-molecule organic films and light-emitting diodes compared with their vacuum-deposited counterparts. Adv. Mater. 19 (10), 1625-1630 (2009).
- Duan, L. Solution processable small molecules for organic light-emitting diodes. J. Mater. Chem. 20, 6392-6407 (2010).
- Kim, S. K. Low-power flexible organic light-emitting diode display device. Adv. Mater. 23, 3511-3516 (2011).
- Kaake, L. G., Barbara, P. F., Zhu, X. Y. Intrinsic charge trapping in organic and polymeric semiconductors: a physical chemistry perspective. J. Phys. Chem. Lett. 1 (3), 628-635 (2010).
- Yersin, H., Rausch, A. F., Czerwieniec, R., Hofbeck, T., Fischer, T. The triplet state of organo-transition metal compounds. Triplet harvesting and singlet harvesting for efficient OLEDs. Coord. Chem. Rev. 255, 2622-2652 (2011).
- Jou, J. H., Kumar, S., Agarwal, A., Lia, T. H., Sahoo, S. Approaches for fabricating high efficiency organic light emitting diodes. J. Mater. Chem. C. 3, 2974-3002 (2015).
- Volz, D. Auto-catalysed crosslinking for next-generation OLED-design. J. Mater. Chem. 22, 20786-20790 (2012).
- Furuta, P. T., Deng, L., Garon, S., Thompson, M. E., Frechet, J. M. J. Platinum functionalized random copolymers for use in solution-processible, efficient, near-white organic light-emitting diodes. J. Am. Chem. Soc. 126 (47), 15388-15389 (2004).
- Biwu, M. New thermally cross-linkable polymer and its application as a hole-transporting layer for solution processed multilayer organic light emitting diodes. Chem. Mater. 19, 4827-4832 (2007).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved