A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Structure-function Studies in Mouse Embryonic Stem Cells Using Recombinase-mediated Cassette Exchange
In This Article
Summary
Proteins often contain multiple domains that can exert different cellular functions. Gene knock-outs (KO) do not consider this functional diversity. Here, we report a recombination-mediated cassette exchange (RMCE)-based structure-function approach in KO embryonic stem cells that allows for the molecular dissection of various functional domains or variants of a protein.
Abstract
Gene engineering in mouse embryos or embryonic stem cells (mESCs) allows for the study of the function of a given protein. Proteins are the workhorses of the cell and often consist of multiple functional domains, which can be influenced by posttranslational modifications. The depletion of the entire protein in conditional or constitutive knock-out (KO) mice does not take into account this functional diversity and regulation. An mESC line and a derived mouse model, in which a docking site for FLPe recombination-mediated cassette exchange (RMCE) was inserted within the ROSA26 (R26) locus, was previously reported. Here, we report on a structure-function approach that allows for molecular dissection of the different functionalities of a multidomain protein. To this end, RMCE-compatible mice must be crossed with KO mice and then RMCE-compatible KO mESCs must be isolated. Next, a panel of putative rescue constructs can be introduced into the R26 locus via RMCE targeting. The candidate rescue cDNAs can be easily inserted between RMCE sites of the targeting vector using recombination cloning. Next, KO mESCs are transfected with the targeting vector in combination with an FLPe recombinase expression plasmid. RMCE reactivates the promoter-less neomycin-resistance gene in the ROSA26 docking sites and allows for the selection of the correct targeting event. In this way, high targeting efficiencies close to 100% are obtained, allowing for insertion of multiple putative rescue constructs in a semi-high throughput manner. Finally, a multitude of R26-driven rescue constructs can be tested for their ability to rescue the phenotype that was observed in parental KO mESCs. We present a proof-of-principle structure-function study in p120 catenin (p120ctn) KO mESCs using endoderm differentiation in embryoid bodies (EBs) as the phenotypic readout. This approach enables the identification of important domains, putative downstream pathways, and disease-relevant point mutations that underlie KO phenotypes for a given protein.
Introduction
It is estimated that mammalian genomes contain about 20,000 protein-coding genes. Alternative splicing and posttranslational modifications further increase the protein repertoire. Proteins have a modular structure1 and often contain multiple interaction domains, which allow their recruitment into different protein complexes and their participation in multiple cellular processes2. One example is the multi-functional protein called p120ctn. p120ctn is encoded by the Ctnnd1 gene and consists of a large central armadillo repeat domain flanked by an N-terminal and a C-terminal region. The armadillo domain of p120ctn binds to a highly conserved juxtamembrane domain of classical cadherins, which are involved in cell-cell adhesion, but it also binds to the transcriptional repressor Kaiso. The N-terminal domain of p120ctn interacts with different kinases, phosphatases, small RhoGTPases, and microtubule-associated proteins3. Interestingly, as a result of alternative splicing, p120ctn isoforms can be generated from four alternative start codons4. p120ctn isoform 1A is the longest, as it is translated from the most-5' start codon and contains the full-length N-terminal segment. In p120ctn isoforms 3 and 4, this N-terminal segment is deleted partially and completely, respectively. Understanding the precise role of proteins (or protein isoforms) and their domains in different cellular functions remains a challenge.
Gene targeting in mESCs enables the study of the function of a protein through the genetic deletion of the corresponding gene and has widely contributed to the identification of developmentally important and disease-relevant genes and pathways. This breakthrough in reverse genetics was the result of advances in the fields of mESC isolation and gene targeting due to homologous recombination5. Homologous recombination is a process in which DNA fragments are exchanged between two similar or identical nucleic moieties after double-stranded (ds) DNA breaks. Normally, HR is inefficient because dsDNA breaks are infrequent. Recently, the efficiency of homology-directed gene targeting could be increased using site-specific nucleases6,7, but unfortunately, these are prone to off-target effects8. A more reliable technique to enable gene targeting is RMCE, which is based on site-specific recombination systems such as Cre/loxP or FLPe/Frt. LoxP and Frt sequence are found in bacteriophage P1 and Saccharomyces cerevisiae, respectively, and consist of 34 bp, including an asymmetric 8 bp sequence that determines the orientation of the site. On the other hand, the orientation of, for instance, two loxP sites within a DNA stretch will determine whether the floxed DNA becomes excised or inversed upon Cre-mediated recombination9. Moreover, Cre can also induce a translocation if two sites are located on different chromosomes. RMCE takes advantage of heterospecific recombination sites that do not cross-react and that are embedded in a genomic locus. In the presence of a donor plasmid that contains a DNA fragment flanked by the same heterospecific sites, the recombinase will insert this DNA fragment into the RMCE-compatible genomic locus because of double-simultaneous translocation (Figure 1). Here, only correctly RMCE-targeted clones can render drug resistance thanks to a promoter on the incoming vector that restores a "trapped," promoter-less Neomycin resistance gene (NeoR) present in the R26 genome of the docking cells (Figure 1)10,11. This results in a very high targeting efficiency, often close to 100%11,12. In conclusion, RMCE-based targeting is highly efficient and can be used for structure-functions studies; however, it requires a pre-engineered genomic locus.
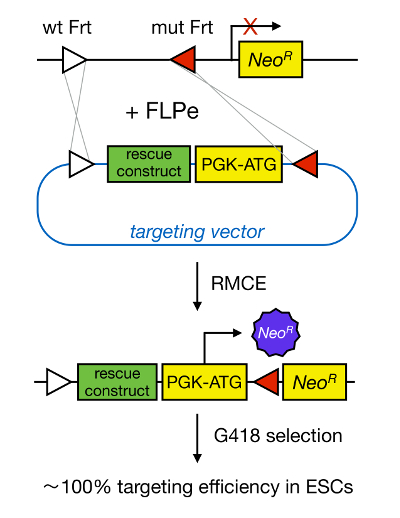
Figure 1. Schematic Representation of RMCE-mediated Targeting. RMCE allows for the exchange of DNA segments from an incoming targeting vector to a defined genomic locus if both harbor two heterospecific Frt sites (depicted by white and red triangles). In addition, the engineered genomic locus contains a promoterless and truncated neomycin-resistance (NeoR) gene. By providing a promoter and start codon in the incoming DNA fragment, only correct recombination events restore neomycin resistance, resulting in high targeting efficiencies. Please click here to view a larger version of this figure.
Genome engineering in mESCs allows for the generation of RMCE-compatible mice. In 1981, two groups succeeded in capturing pluripotent cells from the inner cell mass (ICM) of blastocysts and in maintaining them in culture13,14. mESCs are capable of self-renewal and differentiation into all types of embryonic and adult cells, including the germ-cell lineage. Therefore, gene targeting in mESCs enables reverse-genetic studies through the development of constitutive or conditional (using the Cre/LoxP system) KO mice. However, the classical way to isolate mouse ES cells is very inefficient. Several major improvements have greatly increased the success rate for deriving mESC lines, including the use of a defined serum-replacement (SR) medium15, alternating between mESC medium containing SR and fetal bovine serum (FBS)16, and the use of pharmacological compounds such as pluripotin or 2i17. Pluripotin, a small synthetic molecule, allows for the propagation of mESCs in an undifferentiated state in the absence of leukemia inhibitory factor (LIF) and mouse embryonic fibroblasts (MEFs)18. Finally, it has been shown that mESCs can be isolated with a very high efficiency (close to 100%) when an SR/FBS medium alternation protocol is combined with LIF and pluripotin19,20. These protocols enable the efficient isolation of RMCE-compatible KO mESCs that can subsequently be used for structure-function studies.
This paper describes a method that enables one to identify the key domains or residues within a protein that are responsible for specific cellular processes. To this end, a pipeline of advanced technologies that enable efficient mESC isolation, targeting vector assembly, and mESC targeting was created. As such, large panels with protein isoforms, domain mutants, and downstream effectors can be introduced in KO mESCs and can be evaluated for their ability to rescue the in vitro KO phenotype.
Protocol
All experiments on mice were conducted according to institutional, national, and European animal regulations.
1. Isolation of RMCE-compatible KO mESCs
- Breed heterozygous KO mice with RMCE-compatible mice, such as ROSALUC mice10 or ROSA26-iPSC mice21. Both RMCE-compatible mice were maintained on a mixed 129/C57BL6/Swiss background.
NOTE: Crossing with heterozygous KO mice is advised to overcome embryonic lethality in homozygous KO mice. - Use PCR to select heterozygous KO mice containing an RMCE cassette in the R26 locus12.
- Breed RMCE-compatible, heterozygous KO mice with heterozygous KO mice and isolate RMCE-compatible, homozygous KO blastocysts.
- Set up time matings in the evening and check for copulation plugs the next morning.
NOTE: Plugs are made of coagulated secretions from the coagulating and vesicular glands of the male. These plugs fill the vagina of the female and persist for 8 - 24 h after breeding. Plugged females are considered to be carrying 0.5 dpc (days post coitum) embryos.- To check for plugs, lift the female by the base of her tail and by examine her vaginal opening for a whitish mass. Spread the lips of the vulva slightly with an angled probe when the plug is difficult to see. Separate plugged females from their male.
- Collect blastocysts at 3.5 dpc.
- Euthanize pregnant females by the approved method (e.g., cervical dislocation). Make a midventral incision and dissect the uterus and oviduct (still attached to each other) using fine scissor and forceps.
- Bend a 26-gauge needle into a 45° angle. Attach a 1-mL syringe filled with M2 medium to this bent needle and use it to flush the blastocysts from the uterus into the lid of a 10-cm dish.
- Insert the needle into the end of the uterus that is closest to the oviduct. Hold the needle in place with fine forceps while pushing the plunger; swelling of the uterus indicates a successful flushing.
- Use a mouth pipette (with a diameter of 100 - 200 µm) to collect all embryos and wash them twice in a drop of fresh M2 medium. Immediately after washing them, transfer the blastocysts to the culture plates (see below).
NOTE: The dissection and handling of blastocysts of should be done in laminar air flow.
- Set up time matings in the evening and check for copulation plugs the next morning.
- Isolate RMCE-compatible KO mESCs
- Prepare a 12-well plate with mitomycin-C-treated DR4 MEFs (see the Table of Materials) one day before the blastocyst isolation.
NOTE: These MEFs were isolated from Tg(DR4)1Jae/J mice that contain four drug-selectable genes and confer resistance to neomycin, puromycin, hygromycin, and 6-thioguanine22.- Coat all culture plates with 0.1% gelatin. Add 0.1% gelatin to the culture plates, incubate for 5 min at 37 °C in 5% CO2, and aspirate the gelatin solution. Seed one quarter of a vial of P2 MEFs in a 12-well plate and grow them in 2 mL of MEF medium (see Table 1, the Table of Materials) to a confluent monolayer19.
- Inactivate them with mitomycin-C (10 µg/mL) for 3 h and wash them twice with phosphate-buffered saline (PBS)19.
- Using a mouth pipette, plate the blastocysts onto gelatinized 12-well plates (1 well/embryo), with the mitomycin-C-treated MEFs in SR-ES cell medium (2 mL/well) supplemented with either 2 µM pluripotin or with 2i (1 µM Erk inhibitor PD0325901 and 3 µM Gsk3 inhibitor CHIR99021). Incubate at 37 °C in 5% CO2.
- Refresh the SR-ES cell medium (supplemented with pluripotin or 2i) every 2 - 3 days.
- Examine each blastocyst under a stereomicroscope at 4.0X magnification and check for hatching and attachment to the MEF layer.
NOTE: When blastocysts hatch, they lose the zona pellucida that encapsulates them. Wells with unattached blastocysts need to be refreshed using the mouth pipetting. - Pick individual ICM outgrowths (using a stereomicroscope) after 10 - 12 days of culture using a P10 pipette with disposable tips. Transfer the outgrowth in approximately 10 µL of medium to a V-shaped, 96-well plate containing 30 µL/well of PBS (at room temperature).
- Add 50 µL of 0.25% trypsin to each well using a multichannel pipette and incubate for 3 min at 37 °C in 5% CO2.
- Add 100 µL of FBS-containing mESC medium; dissociate the ICM outgrowths into single cells by pipetting 10-15 times; and transfer the dissociated cells to mitomycin-C-treated, 96-well MEF plates that were prepared one day before the ICM colonies are picked.
- From this step onwards, omit pluripotin or 2i from the mESC medium. On the next day, change the medium from FBS- to SR-containing mESC medium (100 µL/well).
- Expand the established mESC lines from 96- to 24-well format19.
- Wash the cells with 200 µL of PBS, add 50 µL of trypsin, and incubate for 5 min at 37 °C in 5% CO. Add 100 µL of FBS-based mESC medium; dissociate by pipetting 10 - 15 times using a multichannel pipette; and transfer the dissociated cells to mitomycin-C-treated, 24-well MEF plates.
- Change to SR-based medium on the next day. Expand the mESCs in a similar fashion from 24- to 6-well format. Make 3 - 4 freezings from a confluent 6-well plate19.
- Identify RMCE-compatible, homozygous KO mESCs using PCR primers for the R26 locus23 and KO allele of choice (in this case, p120ctn; PCRs for p120ctn null and floxed alleles were described before12).
- Prepare a 12-well plate with mitomycin-C-treated DR4 MEFs (see the Table of Materials) one day before the blastocyst isolation.
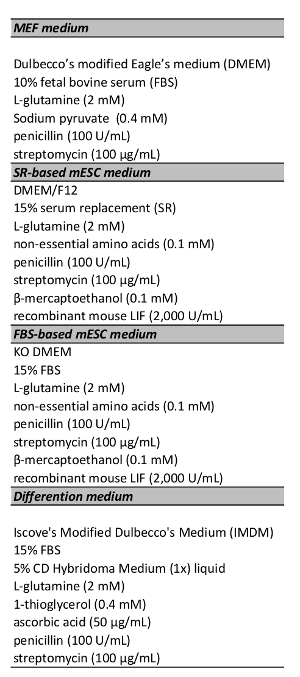
Table 1. Culture Media. All media were stored at 4 °C and warmed to 37 °C 30 min before use.
2. Generation of an RMCE-compatible Targeting Vector Using Recombination Cloning
- Clone the rescue constructs into recombination-compatible vectors using restriction enzyme (RE)-based or PCR-based24 cloning techniques. Make sure that the cDNAs contain a stop codon.
- Design AttB-tagged primers24. Ensure that the forward primer contains the following elements: a GGGG stretch, an AttB1 site, a linker, a Kozak sequence, and about 25 nucleotides of rescue cDNA (starting with its ATG). Make sure that the reverse primer has a similar composition: a GGGG stretch, an AttB2 site, a linker, and about 25 nucleotides of rescue cDNA (reverse complement).
- Amplify the rescue cDNA via PCR to obtain AttB-flanked cDNA.
- Perform a 10-µL BP reaction with 100 ng of AttB-flanked cDNA and 150 ng of recombination-compatible donor vector, which contains a kanamycin-resistance gene.
- Transform 5 µL of the BP mixtures in heat-shock-competent MC1061 Escherichia coli (E. coli) bacteria (similar to those described in step 2.3).
- Identify colonies containing the correct rescue cDNA-containing vectors (similar to those described in step 2.4)
- Perform a 10-µL LR reaction using 100 ng of rescue cDNA-containing vector; 150 ng of Cre-excised pRMCE-DV1 vector11 (LMBP 8195); and 2 µL of recombinase mix, which contains a phage-encoded integrase and excisionase and a bacterial integration host factor. Incubate for 2 h at 25 °C.
NOTE: An LR reaction is a recombination reaction in which an entry clone containing attL sites and a destination vector containing attR sites are recombined by the LR clonase enzyme mix. This results in an expression clone containing attB sites flanking the gene of interest. - Transform 5 µL of the LR mixtures in heat-shock-competent MC1061 E. coli bacteria.
- Add 5 µL of LR mixtures to a ribbed, skirted, 2-mL screw-cap tube with 40 µL of heat-shock-competent E. coli bacteria and incubate for 20 min on ice. Incubate for 5 min at 37 °C.
- Add 1 mL of Luria broth (LB) medium and incubate for 1 h at 37 °C. Plate 50 µL on ampicillin (Amp; 100 µg/mL)-containing agar plates and grow overnight at 37 °C.
- Identify the colonies with the correct targeting vector.
- Pick 5 colonies randomly using a p200 tip. Transfer the tip to a glass test tube containing 2 - 5 mL of LB medium and grow overnight at 37 °C.
- Extract the plasmid DNA from the bacterial cultures using commercially available kits.
- Validate them using RE digests and sequencing. Cut 0.5 - 2 µg of plasmid using 0.2 µL of RE (20 U/µL) and 1 µL of the corresponding 10x buffer in a 10-µL reaction. Incubate for at least 1 h at 37 °C and separate on a 1% agarose gel. Select for colonies with the predicted pattern of DNA fragments.
- Analyze the confirmed vectors (50 ng/µL) with Tlox F (ATC ATG TCT GGA TCC CCA TC) and IRES R (GGG GCG GAA TTC GAT ATC AAG) primers (5 pmol/µL) using Sanger sequencing.
3. RMCE-mediated mESC Targeting of Rescue Constructs to the R26 Locus
- Start a culture of RMCE-compatible KO mESCs and passage them at least twice on MEFs in FBS-based mESC medium. Split the mESCs on a gelatinized 6-well plate.
- On the next day, refresh the cells, at about 50% confluency, with 1.5 mL of FBS-based mESC medium and transfect the mESCs with a Cre-excised pRMCE-DV1 targeting vector containing rescue cDNA.
- Make a DNA mix. Add 1 µg of targeting vector and 1 µg of FLPe-expression plasmid25 to 250 µL of pure DMEM medium.
- Make a lipofection mix. Add 7 µL of lipofection-based transfection reagent (e.g., Lipofectamine 2000) to 250 µL of pure DMEM medium and incubate for 5 min at room temperature (RT).
NOTE: Similar RMCE targeting efficiencies were obtained using other lipofection-based reagents (e.g., Lipofectamine LTX and Effectene). - Mix the DNA mix with the lipofection mix and incubate for 20 min at RT. Pipette the transfection mixture onto the refreshed mESCs. Swirl gently and leave overnight.
- One day after transfection, split all mESCs from the 6-well plate to a 10-cm culture dish with a confluent layer of DR4 MEFs and 10 mL of FBS-based mESC medium.
- Two days after transfection, select mESC clones with the correct FLPe-mediated cassette exchange by adding 0.2 mg/mL G418 (100x) to the medium.
NOTE: Make a kill curve for each batch of G418 to identify the lowest concentration of G418 that kills all mESCs. - Refresh the mESCs daily with G418-containing mESC medium. Colonies should appear after 7 - 10 days, so pick and expand these as per step 1.4.
- Confirm the correct clones via PCR11.
4. Differentiation of mESCs in Embryoid Bodies (EBs)
- Start a culture of KO mESCs with R26-driven rescue constructs and passage them at least twice on MEFs in FBS-based mESC medium. Passage the mESCs once on gelatinized 6-well plates to get rid of the MEFs.
- Wash with PBS and trypsinize the nearly-confluent mESC cultures. Plate dissociated mESCs in different dilutions (1/20 and 1/40) onto non-adherent, bacterial-grade petri-dishes in differentiation medium.
- Allow EBs to form in these dishes for 30 days. Refresh the medium every 2 - 3 days using the following procedure: transfer the EB suspension to a 50-mL tube, let the EBs settle by gravity, remove the supernatant, add fresh medium, and transfer the EB suspension back to a bacterial-grade dish.
- Analyze the targeted mESCs and EBs by immunofluorescence and transmission electron microscopy using protocols that were described previously12.
Results
The procedure to isolate RMCE-compatible KO mESC lines is depicted in Figure 2. Two consecutive breedings are required to obtain RMCE-compatible KO blastocysts. First, heterozygous KO mice are crossed with RMCE-compatible mice to obtain RMCE-compatible, heterozygous KO mice. These mice are then used for timed matings with other heterozygous KO mice to obtain 3.5-dpc, RMCE-compatible, homozygous KO blastocysts. The chance of obtaining such an embryo is one in eight, as pre...
Discussion
Our mESC isolation method is user-friendly and does not require advanced skills or equipment, such as microsurgery of blastocysts. Thus, this technology is accessible to a large proportion of the scientific community. Anyone with basic cell culture experience can propagate ICM outgrowths and establish mESCs lines. However, the flushing and handling of blastocysts requires some practice. A mouth pipette is used to transfer blastocysts and consists of a micropipette, a micropipette holder, tubing, and an aspirator mouthpie...
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Jinke D'Hont, Frederique Van Rockeghem, Natalie Farla, Kelly Lemeire, and Riet De Rycke for their excellent technical support. We also thank Eef Parthoens, Evelien Van Hamme, and Amanda Goncalves from the Bioimaging Core Facility of the Inflammation Research Center for their expert assistance. We acknowledge members of our research group for valuable discussions. This work was supported by the Belgian Science Policy (Belspo Interuniversity Attraction Poles - Award IAP VII-07 DevRepair; https://devrepair.be), by the Queen Elisabeth Medical Foundation, Belgium (GSKE 2008-2010; http://www.fmre-gske.be), and by the Concerted Research Actions (GOA 01G01908) of Ghent University, Belgium (http://www.ugent.be/en/ghentuniv). SG is a postdoctoral fellow of the Flanders Research Funds (FWO-V).
Materials
| Name | Company | Catalog Number | Comments |
| ROSALUC Mice | made in house | frozen sperm available upon request | |
| R26-iPSC mice | made in house | frozen sperm available upon request | |
| Vessel probe | Fine Science Tools | 10160-13 | to check for copulation plugs |
| M2 medium | Sigma-Aldrich | M7167 | make aliquots and store at -20 °C |
| Fine forceps (Dumont #5 Standard tip Student forceps) | Fine Science Tools | 11251-10 | spray with 70% EtOH before use (do not autoclave) |
| 23 G needles | Fine-ject | 8697 | |
| 1-mL syringes | Soft-ject | 6680 | |
| 60-mm bacterial grade plates (for flushing) | Gosselin | BB60-01 | |
| Mouth pipette | made in house | see discussion | |
| Mouse embryonic fibroblasts (MEFs, TgN (DR4)1 Jae strain) | ATTC | SCRC-1045 | |
| TgN (DR4)1 Jae mice | The Jackson Laboratory | 3208 | |
| Mitomycin C | Sigma-Aldrich | M0503 | |
| Phosphate buffered saline (PBS) without calcium or magnesium | Gibco | 14190-094 | |
| Tg(DR4)1Jae/J mice | JAX | 3208 | mice that contain four drug-selectable genes and DR4 MEFS confers resistance to neomycin, puromycin, hygromycin and 6-thioguanine |
| 0.1% Gelatin | Sigma-Aldrich | G1393 | Dissolve 0.5 g in 500 mL distilled water, autoclave and store at 4 °C. |
| Trypsin (0.25%) | Gibco | 25200-056 | |
| 2 μM pluripotin | Cayman Chemical | 10009557 | |
| pRMCE-DV1 | BCCM/LMBP collection | LMBP 08870 | public available from the BCCM/LMBP collection (http://bccm.belspo.be) |
| cre-excised pRMCE-DV1 | BCCM/LMBP collection | LMBP 08195 | public available from the BCCM/LMBP collection (http://bccm.belspo.be) |
| pCAG-FlpE-IRES-Puro-pA | Addgene | 20733 | |
| heat-shock competent DH5α bacteria | made in house | ||
| Gateway pDONR221 vector | Thermo Fisher | 12536-017 | contains kanamycin-resistance gene |
| BP clonase II mix | Thermo Fisher | 11789-020 | |
| LR clonase II mix | Thermo Fisher | 11791-020 | |
| Luria Broth (LB) | |||
| Ampicillin | |||
| Applied Biosystems 3730XL DNA Analyzer | Thermo Fisher | 3730XL | |
| G418 | Thermo Fisher | 11811-023 | |
| Lipofectamine 2000 transfection reagent | Thermo Fisher | 11668027 | |
| Lipofectamine LTX transfection reagent | Thermo Fisher | 15338100 | |
| Effectene transfection reagent | Qiagen | 301425 | |
| GATEWAY pENTR 1A vector | Thermo Fisher | A10462 | recombination-compatible vector |
| mouse monoclonal anti-p120ctn antibody | BD Transduction Laboratories | 610134 | |
| mouse monoclonal anti-Ecadherin antibody | BD Transduction Laboratories | 610181 | |
| General equipment | |||
| Sterile dissection tools | fine scissors and forceps for dissecting the uterus | ||
| Sterile pipettes: 5 mL, 10 mL and 25 mL | |||
| 15-mL and 50-mL conical centrifuge tubes | |||
| 96-well culture plates V-shaped bottom and flat bottom) | |||
| Culture dishes: 24 wells, 12 wells and 6 wells | |||
| Multichannel pipettes (to pipette 30, 50, 100 and 200 μL) | |||
| Sterile multichannel reservoirs | |||
| Access to a laminar air flow | |||
| Access to an incubator at 37 °C with 5% CO2 | |||
| Access to an inverted microscope | |||
| Access to a bench-top centrifuge | |||
| Access to a stereo microscope with transmitted-light | |||
| Culture media | |||
| MEF Medium | stored at 4 °C; warm 30 min at 37 °C before use | ||
| Dulbecco’s modified Eagle’s medium (DMEM) | Gibco | 41965-062 | |
| 10% fetal bovine serum (FBS) | Sigma-Aldrich | F-7524 | |
| L-glutamine (2 mM) | Gibco | 25030-024 | |
| Sodium pyruvate (0.4 mM) | Gibco | 11360-039 | |
| penicillin (100 U/mL) | Gibco | 15140-122 | |
| streptomycin (100 µg/mL) | Gibco | 15140-122 | |
| SR-based mESC medium | stored at 4 °C; warm 30 min at 37 °C before use | ||
| DMEM/F12 | Gibco | 31330-038 | mixed in a 1:1 ratio |
| 15% knock-out serum replacement (SR) | Gibco | 10828–028 | |
| L-glutamine (2 mM) | Gibco | 25030-024 | |
| 0.1 mM non-essential amino acids | Gibco | 11140-050 | |
| penicillin (100 U/mL) | Gibco | 15140-122 | |
| streptomycin (100 µg/mL) | Gibco | 15140-122 | |
| β-mercaptoethanol (0.1 mM) | Sigma-Aldrich | M 3148 | |
| 2,000 U/mL recombinant mouse LIF | (IRC/VIB Protein Service facility) | ||
| FBS-based mESC medium (similar to SR-based mESC medium) | stored at 4°C; warm 30 min at 37°C before use | ||
| Knockout DMEM | Gibco | 10829-018 | |
| 15% FBS | Hyclone | SH30070.03E | |
| Differention Medium | stored at 4 °C; warm 30 min at 37 °C before use | ||
| Iscove's Modified Dulbecco's Medium (IMDM) | Gibco | 21980-032 | |
| 15% FBS | Hyclone | SH30070.03E | |
| 5% CD Hybridoma Medium(1x) liquid | Gibco | 11279-023 | |
| 2 mM L-glutamine | Gibco | 25030-024 | |
| 0.4 mM 1-thioglycerol | Sigma-Aldrich | M-6145 | |
| 50 μg/mL ascorbic acid | Sigma-Aldrich | A-4544 | |
| penicillin (100 U/mL) | Gibco | 15140-122 | |
| streptomycin (100 µg/mL) | Gibco | 15140-122 | |
| 2i | |||
| 1 μM Erk inhibitor PD0325901 | Axon Medchem | Axon 1408 | |
| 3 μM Gsk3 inhibitor CHIR99021 | Axon Medchem | Axon 1386 |
References
- Gul, I. S., Hulpiau, P., Saeys, Y., van Roy, F. Metazoan evolution of the armadillo repeat superfamily. Cell Mol Life Sci. , (2016).
- Valenta, T., Hausmann, G., Basler, K. The many faces and functions of beta-catenin. EMBO J. 31, 2714-2736 (2012).
- Pieters, T., van Hengel, J., van Roy, F. Functions of p120ctn in development and disease. Front Biosci. 17, 760-783 (2012).
- Keirsebilck, A., et al. Molecular cloning of the human p120ctn catenin gene (CTNND1): Expression of multiple alternatively spliced isoforms. Genomics. 50, 129-146 (1998).
- Capecchi, M. R. Gene targeting in mice: functional analysis of the mammalian genome for the twenty-first century. Nat Rev Genet. 6, 507-512 (2005).
- Wang, H., et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 153, 910-918 (2013).
- Yang, H., et al. One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering. Cell. 154, 1370-1379 (2013).
- Fu, Y., et al. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat Biotechnol. 31, 822-826 (2013).
- Branda, C. S., Dymecki, S. M. Talking about a revolution: The impact of site-specific recombinases on genetic analyses in mice. Dev Cell. 6, 7-28 (2004).
- Sandhu, U., et al. Strict control of transgene expression in a mouse model for sensitive biological applications based on RMCE compatible ES cells. Nucleic Acids Res. 39, 1 (2010).
- Haenebalcke, L., et al. Efficient ROSA26-based conditional and/or inducible transgenesis using RMCE-compatible F1 hybrid mouse embryonic stem cells. Stem Cell Rev. 9, 774-785 (2013).
- Pieters, T., et al. p120 Catenin-Mediated Stabilization of E-Cadherin Is Essential for Primitive Endoderm Specification. PLoS Genet. 12, e1006243 (2016).
- Evans, M. J., Kaufman, M. H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 292, 154-156 (1981).
- Martin, G. R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 78, 7634-7638 (1981).
- Cheng, J., Dutra, A., Takesono, A., Garrett-Beal, L., Schwartzberg, P. L. Improved generation of C57BL/6J mouse embryonic stem cells in a defined serum-free media. Genesis. 39, 100-104 (2004).
- Bryja, V., et al. An efficient method for the derivation of mouse embryonic stem cells. Stem Cells. 24, 844-849 (2006).
- Ying, Q. L., et al. The ground state of embryonic stem cell self-renewal. Nature. 453, 519-523 (2008).
- Chen, S., et al. Self-renewal of embryonic stem cells by a small molecule. Proc Natl Acad Sci U S A. 103, 17266-17271 (2006).
- Pieters, T., et al. Efficient and User-Friendly Pluripotin-based Derivation of Mouse Embryonic Stem Cells. Stem Cell Rev. 8, (2012).
- Yang, W., et al. Pluripotin combined with leukemia inhibitory factor greatly promotes the derivation of embryonic stem cell lines from refractory strains. Stem Cells. 27, 383-389 (2009).
- Haenebalcke, L., et al. The ROSA26-iPSC Mouse: A Conditional, Inducible, and Exchangeable Resource for Studying Cellular (De)Differentiation. Cell Rep. 3, (2013).
- Tucker, K. L., Wang, Y., Dausman, J., Jaenisch, R. A transgenic mouse strain expressing four drug-selectable marker genes. Nucleic Acids Res. 25, 3745-3746 (1997).
- Nyabi, O., et al. Efficient mouse transgenesis using Gateway-compatible ROSA26 locus targeting vectors and F1 hybrid ES cells. Nucleic Acids Res. 37, 55 (2009).
- Katzen, F. Gateway((R)) recombinational cloning: a biological operating system. Expert Opin Drug Discov. 2, 571-589 (2007).
- Schaft, J., Ashery-Padan, R., vander Hoeven, F., Gruss, P., Stewart, A. F. Efficient FLP recombination in mouse ES cells and oocytes. Genesis. 31, 6-10 (2001).
- Spencer, H. L., et al. E-cadherin inhibits cell surface localization of the pro-migratory 5T4 oncofetal antigen in mouse embryonic stem cells. Mol Biol Cell. 18, 2838-2851 (2007).
- Stryjewska, A., et al. Zeb2 Regulates Cell Fate at the Exit from Epiblast State in Mouse Embryonic Stem Cells. Stem Cells. , (2016).
- Nagy, A., Gertsenstein, M., Vintersten, K., Behringer, R. . Manipulating the mouse embryo: A laboratory manual. , (2003).
- van den Berghe, V., et al. Directed migration of cortical interneurons depends on the cell-autonomous action of Sip1. Neuron. 77, 70-82 (2013).
- Li, J., et al. The EMT transcription factor Zeb2 controls adult murine hematopoietic differentiation by regulating cytokine signaling. Blood. , (2016).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved