A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Using an Adapted Microfluidic Olfactory Chip for the Imaging of Neuronal Activity in Response to Pheromones in Male C. Elegans Head Neurons
In This Article
Summary
The use of an adapted "olfactory chip" for the efficient calcium imaging of C. elegans males is described here. Studies of male exposure to glycerol and a pheromone are also shown.
Abstract
The use of calcium indicators has greatly enhanced our understanding of neural dynamics and regulation. The nematode Caenorhabditis elegans, with its completely mapped nervous system and transparent anatomy, presents an ideal model for understanding real-time neural dynamics using calcium indicators. In combination with microfluidic technologies and experimental designs, calcium-imaging studies using these indicators are performed in both free-moving and trapped animals. However, most previous studies utilizing trapping devices, such as the olfactory chip described in Chronis et al., have devices designed for use in the more common hermaphrodite, as the less common male is both morphologically and structurally dissimilar. An adapted olfactory chip was designed and fabricated for increased efficiency in male neuronal imaging with using young adult animals. A turn was incorporated into the worm loading port to rotate the animals and to allow for the separation of the individual neurons within a bilateral pair in 2D imaging. Worms are exposed to a controlled flow of odorant within the microfluidic device, as described in previous hermaphrodite studies. Calcium transients are then analyzed using the open-source software ImageJ. The procedure described herein should allow for an increased amount of male-based C. elegans calcium imaging studies, deepening our understanding of the mechanisms of sex-specific neuronal signaling.
Introduction
Microfluidic devices provide increased access to precisely controlled environments, wherein animals, such as the nematode C. elegans, can be experimentally manipulated1. These studies include behavioral assays, calcium imaging studies, or even screenings for specific phenotypes, resulting in more exact measurements of experimental outcomes1,2,3,4,5,6. Microfluidics provide small-scale liquid conditions through which detailed experiments can be run while utilizing minimal amounts of reagents. There is a constant production of new microfluidic device designs, and the use of each varies, from arenas that allow for the natural sinusoidal motion of C. elegans in behavioral assays and neural imaging studies, to trap devices used in neural imaging and olfactory studies, to devices that allow for high-throughput phenotypic analysis in genetic screens4,5,6,7. Following the fabrication of a master mold, microfluidic devices are inexpensive to construct—given the reusability of the master—and easy to use, allowing for rapid data generation via high-throughput studies. The fabrication of devices using polymers such as polydimethylsiloxane (PDMS) allows for the creation of new devices within hours.
Calcium imaging studies use genetically encoded calcium indicators (GECIs) expressed in target cells to measure the neural dynamics of those cells in real time8,9,10,11. The transparent nature of C. elegans allows for the recording of the fluorescent levels of these proteins in live animals. Traditionally, GECIs rely on the green fluorescent protein (GFP)-based sensor GFP-Calmodulin-M13 Peptide (GCaMP), although more recent studies have adapted these sensors to allow for better signal-to-noise ratios and red-shifted excitation profiles. Following the development of GCaMP3, proteins with these specifications have varied, including sensors such as GCaMP6s and GCaMP6f (slow and fast fluorescence off-rates, respectively), as well as RFP-Calmodulin-M13 Peptide (RCaMP), which has a red-shifted activation profile. The combination of these GECIs with C. elegans cell-specific gene promoter sequences can target cells of interest, particularly sensory neurons12,13,14,15,16.
While the ease of C. elegans use in microfluidic studies is apparent, almost all studies have focused on hermaphrodites. Despite males only accounting for 0.01-0.02% of the wild type population, invaluable findings can arise from their characterization. While the physical connectome of the hermaphrodite nervous system has been fully mapped for decades17, the male connectome remains incomplete, especially in the head region of the animal18. The use of calcium imaging in males will help to generate an understanding of the male nervous system and the differences that arise between the two sexes. The smaller size of C. elegans adult males prevents effective and reliable trapping in the loading ports of traditional olfactory devices designed for larger hermaphrodites. To address this, a modified version of the Chronis Olfactory Chip19 was developed with a narrower loading port, a lower channel height, and turns in the worm loading port (which rotate the animal), allowing for the visualization of bilateral left/right neuronal pairs. This design permits: (1) the effective trapping of young adult males, (2) a more reliable orientation of the animal for the visualization of both members of bilateral paired neurons, and (3) the precise imaging of neural activity in male neurons.
Increasingly, studies show that C. elegans males respond differently than hermaphrodites to a variety of ascarosides (ascr), or nematode pheromones20,21,22,23,24. Therefore, developing an understanding of the neural dynamics and representations within the male connectome has become even more pertinent. Male C. elegans contain 87 sex-specific neurons not present in the hermaphrodite25,26, altering the connectome in as-yet undetermined ways. Being able to image these unique neural dynamics will allow us to better understand sex-specific responses and neural representations.
This protocol describes the use of a male-adapted olfactory chip for the neural imaging of male C. elegans chemosensation. The nociceptive neuron ASH responds reliably to 1 M glycerol in males, consistent with previous hermaphroditic studies27. Exposure to ascarosides may elicit responses that are variable from animal to animal, requiring a larger number of animals to be tested. The response of the male-specific CEM neurons has previously been shown, through both electrophysiology and calcium imaging studies, to respond variably to ascaroside #323.
Protocol
1. Device Fabrication
NOTE: See reference1.
NOTE: Silicon master molds were fabricated using standard photolithographic techniques for patterning SU-8 photoresist on a silicon master1,7. Photomasks for wafer patterning were printed at 25,000 dpi. The male-adapted device features a Chronis Olfactory Chip design19 with a change in the worm loading port, adapting a design obtained from M. Zimmer (personal correspondence, 2016). A turn is included to control the rotation of the animals. The width of the worm loading port channel is narrowed to 50 μm. All channels are 32 μm tall. Once a silicon master mold is available to the user, the user can follow the subsequent protocol, as described previously1.
- Mix PDMS base and curing agent at a 10:1 ratio by weight.
- Mix thoroughly with transfer pipettes.
- Degas the mixture in a vacuum desiccator for 1 h, until all visible bubbles are removed.
- Pour the mixture onto a silicon mold master in a 150 mm diameter dish until it is 5 mm thick (100 g). Use a Pasteur pipette to remove any bubbles or dust that have been introduced to the mixture.
- Bake at 65 °C for at least 3 h, or overnight.
- Cut the PDMS away from the mold using a scalpel and cut the separate devices apart using a razor blade.
- Punch inlet and outlet holes with a 1 mm dermal punch.
- Flush the holes with dH2O, ethanol, and again with dH2O to remove particles from the punches. Dry the device in an air stream pulse.
- Clean both channel sides and the top side of the device with adhesive tape, removing any dust or debris remaining on the device to allow for successful bonding.
- Plasma-bond the device, channel-side down, to a no. 1 cover glass.
- Expose cover glass and device (channel-side up) to air plasma using conditions that allow for proper bonding, such as 100 W for 30 s or 24 W for 60 s.
NOTE: Settings can be adjusted to improve the bonding efficiency. The plasma-bonding conditions are not as critical as proper cleaning when attempting to improve the bonding efficiency. An insufficiently cleaned device will not bond, even under ideal plasma conditions. - Invert the cover glass onto the channel side of the device and press down with the thumb for 5 s.
- Expose cover glass and device (channel-side up) to air plasma using conditions that allow for proper bonding, such as 100 W for 30 s or 24 W for 60 s.
2. Buffer Preparation
- Dilute 1x S Basal (100 mM NaCl and 0.05 M KPO4, pH 6.0) from a sterile 10x stock.
- Dilute 1 M tetramisole stock to a final concentration of 1 mM in 1x S Basal for all buffer solutions.
- Add fluorescein to both the "flow control" and "buffer" reservoirs.
- Create a 100-mg/mL stock of fluorescein in 1x S Basal.
- Dilute the stock to final concentrations of 1 µg/mL in the flow control and 0.1 µg/mL in the buffer.
- Create the stimuli.
- Dilute glycerol to a final concentration of 1 M in 1X S Basal.
- Dilute ascaroside #3 (ascr#3) to a final concentration of 1 µM into 1X S Basal.
3. Device Setup
NOTE: See1.
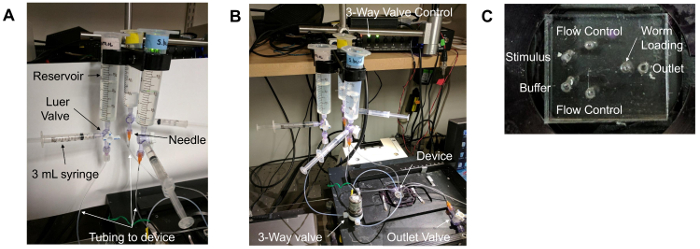
Figure 1. Microfluidic device setup. (A) Reservoirs and tubing. A 30 mL syringe without a plunger serves as the "reservoir." This is attached to a Luer valve with three flow options. One outlet is connected to a 3 mL syringe with a plunger, while the other is connected to a needle (orange) that is inserted into the tubing that connects to the microfluidic device. (B) The overall setup of the microfluidic imaging experiment. The device is placed on a stage of an inverted epifluorescence microscope, above the objective lenses. The "flow control" buffer travels through a 3-way valve that is controlled by a unit on the shelf above the setup. Lines containing buffers are then inserted into the appropriate device ports. (C) The ports of the microfluidic device. The "flow control" ports flank the other inlet ports: the "stimulus" and "buffer" ports. The "outlet" port is the right-most port. Due to the location of the worm loading arena, the "worm loading" port is the central-most port on the device. Please click here to view a larger version of this figure.
- Prepare three fluid reservoirs by attaching a 30 or 60 mL syringe to 3-way Luer valve, with a 3 mL syringe and needle attached to the Luer valve as well (as in Figure 1A). Connect the needle to tubing that extends to the microfluidic device (as in Figure 1A-B).
- Remove the air bubbles from the reservoir and tubing.
- Fill the 3 mL syringe with attached tubing with 1x S Basal and insert it into the outlet port.
- Gently apply pressure to the syringe until the buffer appears at the top of the inlet holes.
- Connect the flow control, buffer, and stimulus tubing to appropriate inlet holes (as in Figure 1B-C), ensuring that liquid drops are present on both the loading port hole and the buffer tubing to be attached.
- Again, gently apply pressure to the syringe that is connected to the outlet port until droplets appear in the worm loading port inlet.
- Insert a solid blocking pin into the worm loading port.
- Remove the syringe from the outlet port and attach the outlet line connected to the house vacuum (-670 Torr).
- Inspect the device for any bubbles in the flow channels, visually and through video confirmation via a software compatible with the camera used, such as the open-source software Micro-Manger. See step 6 for tips on using Micro-Manager.
- If any bubbles are present, wait for them to dislodge or be absorbed into the PDMS wall prior to loading any animals; the presence of bubbles will disturb the proper flow of fluids through the device.
- Using a GFP filter, confirm proper flow dynamics within the device prior to worm loading by actuating the 3-way valve and observing the switching of buffers.
- Determine the proper flow dynamics: observe the fluorescein present in the flow control and buffer solutions (Figure 2D-2E) changing when the flow control value is changed by pressing the control button corresponding to the 3-way valve on the perfusion controller software (Figure 1B).
- After opening Micro-Manager, click on "Live" to observe a live image of the device. Turn on the fluorescent light source to observe the flow of buffers in the device (Figure 2D-2E).
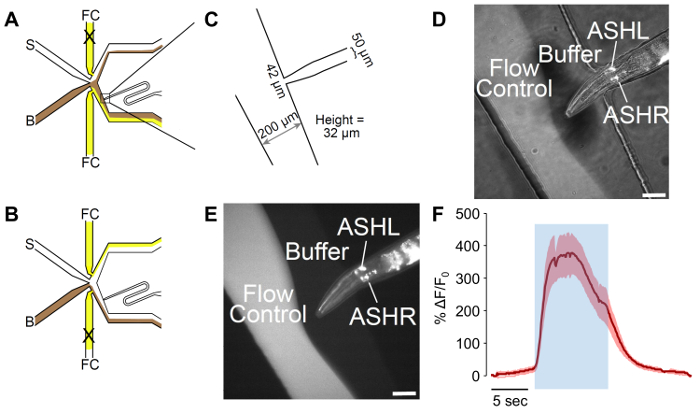
Figure 2: A male-adapted microfluidic olfactory chip. (A) The flow patterns of the device when the worm is exposed to buffer. Buffer (B) is shown in brown, and flow control (FC) is shown in yellow, with stimulus (S) in white. The worm loading port has been adapted to include a curve, which allows for better control of worm orientation. (B) The flow patterns of the device when the worm is exposed to stimulus. Buffer (B) is shown in brown, and flow control (FC) is shown in yellow, with stimulus (S) in white. (C) Measurements of the adapted device as fabricated. The worm loading port ends in a 42 µm opening, with a 50 µm channel designed for the male width. The measured height of the channels is 32 µm, despite a target of 25 µm in the design. (D-E) A trapped male expressing psra-6::GCaMP3. The sra-6 promoter is not ASH-specific, and some expression may be observed in the ASI neuron, although no calcium transients were observed in ASI. The image is (D) a combination of bright-field and fluorescent illumination, while (E) is fluorescent only. The scale bars denote 42 µm. (F) The ASH neuron responds to 1 M glycerol stimulation with robust neural activity. The blue area denotes the time of the 1 M glycerol stimulus. The shaded region denotes the standard error, with n = 20 pulses from seven worms. The red traces denote depolarizing responses. The Y-axes show ΔF/F0. The scale bar denotes 5 s. Please click here to view a larger version of this figure.
4. Animal Preparation
NOTE: See reference23.
- Imaging ASH responses to 1 M glycerol.
- Place approximately 20 C. elegans males that are positive for psra-6::GCaMP3 array expression onto a nematode growth medium (NGM) agar plate seeded with a lawn of OP50 E. coli. Use the expression of fluorescent GECI and/or a co-injection marker for the identification of array-positive animals.
NOTE: Array positive animals will fluoresce according to the GECI used (i.e., animals expressing GCaMP will fluoresce green under blue-light stimulation, while RCaMP animals will fluoresce red under green-light stimulation). Co-injection markers can range from other fluorescent proteins, such as GFP and RFP, to phenotypic markers, such as rol-6, or can rescue a dominant phenotype, such as the pha-1 mutation28.- If picking immediately prior to the assay, pick young adult males. If picking the day prior to the assay, pick L4 larval males.
- Place approximately 20 C. elegans males that are positive for psra-6::GCaMP3 array expression onto a nematode growth medium (NGM) agar plate seeded with a lawn of OP50 E. coli. Use the expression of fluorescent GECI and/or a co-injection marker for the identification of array-positive animals.
- Imaging the CEM responses to 1 µM ascr#3.
- Pick approximately 20 L4 C. elegans males (fkEx98[ppkd-2::GCaMP::SL2::dsRED + pBX-1]; pha-1(e2123ts); him-5(e1490); lite-1(ce314)) that are positive for dsRed co-injection marker expression.
NOTE: dsRed expression within the ray neurons of the male tale is easier to observe and confirm than GCaMP expression within the four CEM neurons. - Isolate these males from hermaphrodites on an NGM agar plate seeded with a lawn of OP50 E. coli for 5-14 h before performing the imaging experiment.
NOTE: Males not isolated for a minimum of 5 h do not behaviorally respond to ascr#3 and therefore may exhibit even fewer calcium transients to the ascaroside than observed here.
- Pick approximately 20 L4 C. elegans males (fkEx98[ppkd-2::GCaMP::SL2::dsRED + pBX-1]; pha-1(e2123ts); him-5(e1490); lite-1(ce314)) that are positive for dsRed co-injection marker expression.
5. Animal Loading
NOTE: See refefence1.
- Pick one worm onto an unseeded NGM agar plate using standard worm maintenance techniques.
- Pick worms by flaming a pick (made of flattened platinum wire), picking bacteria onto the pick, and "dabbing" a worm to pick it up. Gently place the worm onto the new plate, allowing it to crawl off on its own.
- Add approximately 5 mL of 1x S Basal to the unseeded plate, such that plate is flooded.
- Draw the worm into a loading syringe (i.e., 3 mL syringe with attached tubing) that has been pre-filled with 1x S Basal.
- Be sure to suck the worm only into the tubing, not all the way into the syringe.
NOTE: If the worm travels into the syringe, it is near impossible to get it back into the tubing.
- Be sure to suck the worm only into the tubing, not all the way into the syringe.
- Turn off the vacuum to stop the flow by turning the outlet Luer valve.
- Remove the solid pin blocking the worm loading port.
- Turn the Luer valve connected to the outlet port (Figure 1B) so that it is venting.
NOTE: Use a live video feed while loading the worm to confirm the location and orientation of the animal (steps 5.8-5.13). - Insert the worm loading tube into the worm loading port.
- Gently apply pressure to the syringe until the worm appears in the loading channel.
- If the worm starts to enter the channel tail-first, pull on the syringe plunger to prevent the worm from entering the channel.
- Switch between applying and reversing pressure until the head enters the channel first.
- Open the vacuum by turning the 3-way Luer valve connected to the outlet port to open it to vacuum instead of atmosphere.
- Manually apply pressure by depressing the syringe plunger to orient and place the worm head such that it is exposed to the buffer flow channel, but not so far that the head can move around freely (Figure 2D-2E).
6. Stimulus and Acquisition
- Using an open-source microscopy software, such as Micro-Manager, record by capturing images as a TIFF stack at 10 frames/s using blue-light excitation (470 nm) for 30 s.
- Set the exposure on the main menu to 100 ms.
- Open "Multi-D Acq." from the main menu of the software. Set the "number" to "300," and the "interval" to "0." Click "Acquire!" to acquire the video.
- Apply a 10 s pulse of the stimulus 5 s after initiating acquisition. Adjust the duration of stimulus application as desired.
- After acquiring 5 s of video, change the 3-way valve controlling the flow control buffer to apply the stimulus to the animal being tested. Click the left-most button on the perfusion controller software (Figure 1B).
- After 10 s of stimulus exposure (this time can be adjusted as desired by the user), alter the flow of buffers by again pressing the left-most button on the perfusion controller software.
- Record under buffer only until the 30-s window is complete to allow the GECI fluorescence to return to baseline.
- Repeat as desired. Wait 30 s between the end of acquisition and the initiation of the next trial.
7. Image Analysis
- Open the TIFF stack with the open-source software, ImageJ, by dragging file into the ImageJ window.
- Click using the cursor and drag to set the region of interest (ROI) around the neuron of interest. Set the region to include the soma of the neuron of interest (as in Figure 3A).
- Plot the z-stack of the fluorescence intensity of the ROI across stacks by clicking Open -> Image -> Stacks -> Plot Z-axis Profile.
- Click "List" in the window that opens. Click Edit -> Copy to copy the values. Paste the values into a spreadsheet program.
- Analyze the background fluorescence for each pulse by dragging the ROI to a region of the worm that does not contain GCaMP expression.
- Perform background subtraction for each pulse by subtracting the background fluorescence value from the neuron fluorescence intensity value.
- Calculate ΔF/F0 for each frame of each pulse.
- Calculate F0 as the average intensity value of the ROI for first 1 s of acquisition (e.g., frames 1-10).
- Calculate ΔF/F0 by dividing the background-subtracted value for the frame of interest by the calculated F0 value.
- Repeat for every neuron imaged and every stimulus pulse.
- For neurons with consistent response profiles, such as ASH, average all pulses for each neuron and calculate the SEM (as in Figure 2F).
- Plot the average ΔF/F0 with SEM over time for each neuron.
NOTE: In this instance, it is common practice to include heatmaps of the individual neuronal responses of each trial as well. In neurons that do not exhibit consistent changes in calcium transients upon exposure to stimuli across repeated stimulations, or in different individuals23, it may be more applicable to show individual pulse traces (as in Figure 4). See the Discussion for details on determining how to display the data.
Results
An example of the overall device setup can be seen in Figure 1A-B. Figure 1A depicts the proper reservoir construction and setup. Figure 1B shows the connections of the reservoirs to the microfluidic device. Figure 1C depicts a microfluidic device with individual ports labeled for clarity.
The ...
Discussion
The male-adapted olfactory chip incorporates a turn into a narrower loading port, which allows for more control of the orientation and for the efficient trapping of male C. elegans. This allows for the visualization of both the left and right members of neuronal bilateral pairs, without the need for z-stacking. This curve leads to an orientation away from vertical 100% of the time in worms where only one bilateral pair is targeted with a fluorescent marker, such as ASH (Figure 2D
Disclosures
The authors have nothing to disclose.
Acknowledgements
We would like to thank Manuel Zimmer for providing us with the initial design file that was adapted for use with males; Frank Schroeder for the synthesis and supply of ascr#3; Ross Lagoy for the insight and assistance with imaging and analysis; and Laura Aurilio for the master fabrication and who, alongside Christopher Chute, contributed to the review of this manuscript. Funding for this work was provided under the National Institutes of Health grant 1R01DC016058-01 (J.S.), the National Science Foundation grant CBET 1605679 (D.R.A.), and the Burroughs Wellcome Career Award at the Scientific Interface (D.R.A.).
Materials
| Name | Company | Catalog Number | Comments |
| Silicon Wafer | University Wafer | 452 | |
| SU-8 2035 | MicroChem | Y111070-0500L1GL | |
| Developer | MicroChem | Y020100-4000L1PE | |
| Wafer Mask | Cad/Art Services | - | Custom order. Printed at 25,000 dpi. |
| Sylgard-184 | Ellsworth Adhesives | 184 SIL ELAST KIT 0.5KG | |
| 1.0 mm Dermal Punches | Acuderm Inc. | P150 | |
| Soft Tubing | Cole-Palmer | EW-06419-01 | |
| Hard Tubing | IDEX Health & Science | 1622 | |
| Pins | New England Small Tube | NE-1027-12 | |
| Blocking Pins | New England Small Tube | 0.415/0.425" OD x .500 Long | Batch PB07027 |
| 3 mL syringes | BD | 309657 | |
| 30 mL syringes | Vitality Medical | 302832 | Used as buffer reservoirs. |
| Stainless Steel Blunt Needle 23 Gauge, Polyprolylene Luer | Component Supply Company | NE-231PL-50 | |
| Stopcocks with Luer connections; 3-way; male lock; 5 flow pattern; non-sterile | Cole-Palmer | EW-30600-07 | |
| Fisherfinest Premium Cover Glass | Fisher Scientific | 12-548-5M | |
| Mercator Control System LF-5 Plasma System | Mercator | LF-5 | |
| Scotch Tape | Scotch | BSN43575 | |
| Series 20 Chamber | Warner Instruments | P-2 | |
| Vacuum Desicator | Bel-Art Scienceware | 420250000 | 24 cm inner diameter. |
| Weigh Boats | Cole-Palmer | EW-01017-27 | |
| Classic Plus Balance | Mettler Toledo | PB1501-S/FACT | |
| Glass Pasteur Pipettes | Cole-Palmer | EW-25554-06 | |
| Transfer pipettes | Genesee Scientific | 30-202 | |
| Oven | Sheldon Manufacturing Inc | 9120993 | Model Number: 1500E. |
| 60 mm, non-vented, sharp edge Petri dishes | TriTech Research | T3308 | |
| Zeiss Axio Observer.A1 | Zeiss | - | |
| Hammamatsu Orca Flash 4.0 Digital CMOS | Hammamatsu | C11440-22CU | |
| Blue Fluorescent Light | Lumencor | SOLA SM6-LCR-SA | 24-30V/7.9A DC. |
| Illumination Adaptor | Zeiss | 423302-0000 | |
| Series 1 and 2 Miniature Inert PTFE Isolation Valve | Parker | 001-0017-900 | 3-way valve for controlling flow. |
| ValveLink8.2® | AutoMate Scientific | 01-18 | Flow Switch Controller |
| Micro Manager | Micro-Manager | - | Free software, can be downloaded at: https://www.micro-manager.org/wiki/Download_Micro-Manager_Latest_Release |
| ImageJ | ImageJ | - | Free software, can be downloaded at: https://imagej.nih.gov/ij/download.html |
| Agar, Bacteriological Grade | Apex | 9012-36-6 | |
| Peptone | Apex | 20-260 | |
| CaCl2 | VWR | BDH0224-1KG | |
| MgSO4 | Sigma-Aldrich | 230391-1kg | |
| Cholesterol | Alfa Aesar | A11470 | |
| Ethanol | Sigma-Aldrich | 270741-4L | |
| Tetramisole | Sigma-Aldrich | L9756-10(G) | Store at 4 °C. |
| Fluorescein | Sigma-Aldrich | FD2000S-250mg | Light Sensitive. Store in photoprotective vials. |
| Glycerol | Sigma-Aldrich | G6279-1L | |
| Ascaroside #3 | - | - | Synthesized in the Schroeder Lab (Cornell University). |
| NaCl | Genesee Scientific | 18-215 | |
| KH2PO4 | BDH | BDH9268.25 | |
| K2HPO4 | J.T. Baker | 3252-025 | |
| ASH GCaMP3 line | - | - | CX10979 (KyEx2865 [psra-6::GCAMP3 @ 100 ng/uL]). Developed in Bargmann lab. Provided from Albrecht Lab library. |
| CEM GCaMP6 line | - | - | JSR49 (FkEx98[ppkd-2::GCaMP::SL2::dsRED + pBX-1]; pha-1(e2123ts); him-5(e1490); lite-1(ce314)). Developed by Robyn Lints. Provided from Srinivasan Lab library. |
| E. coli (OP50) | Caenorhabditis Genetics Center | OP50 | |
| "Reservoir" | - | - | To create a Reservoir: A "30 mL syringe", is connected to a "Stopcock with Luer connections; 3-way; male lock; 5 flow pattern; non-sterile", which is connected to a "3 mL syringe" and a "Stainless Steel Blunt Needle 23 Gauge, Polyprolylene Luer". The "Stainless Steel Blunt Needle 23 Gauge, Polyprolylene Luer" is then inserted into "Soft Tubing" approximately 1/3 of the way down the needle. |
References
- Lagoy, R. C., Albrecht, D. R. Microfluidic Devices for Behavioral Analysis, Microscopy, and Neuronal Imaging in Caenorhabditis elegans. Methods Mol Biol. 1327, 159-179 (2015).
- Ben-Yakar, A., Chronis, N., Lu, H. Microfluidics for the analysis of behavior, nerve regeneration, and neural cell biology in C. elegans. Curr Opin Neurobiol. 19 (5), 561-567 (2009).
- Chronis, N. Worm chips: Microtools for C. elegans biology. Lab on a Chip. 10 (4), 432-437 (2010).
- Lee, H., Crane, M. M., Zhang, Y., Lu, H. Quantitative screening of genes regulating tryptophan hydroxylase transcription in Caenorhabditis elegans using microfluidics and an adaptive algorithm. Integr Biol (Camb). 5 (2), 372-380 (2013).
- Lockery, S. R., et al. A microfluidic device for whole-animal drug screening using electrophysiological measures in the nematode C. elegans. Lab Chip. 12 (12), 2211-2220 (2012).
- Mondal, S., et al. Large-scale microfluidics providing high-resolution and high-throughput screening of Caenorhabditis elegans poly-glutamine aggregation model. Nat Commun. 7, 13023 (2016).
- Larsch, J., Ventimiglia, D., Bargmann, C. I., Albrecht, D. R. High-throughput imaging of neuronal activity in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 110 (45), E4266-E4273 (2013).
- Akerboom, J., et al. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front Mol Neuro. 6, 2 (2013).
- Badura, A., Sun, X. R., Giovannucci, A., Lynch, L. A., Wang, S. S. H. Fast calcium sensor proteins for monitoring neural activity. Neurophotonics. 1 (2), 025008 (2014).
- Tatro, E. T. Brain-wide imaging of neurons in action. Front Neural Circuits. 8, 31 (2014).
- Tian, L., et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 6 (12), 875-881 (2009).
- Greene, J. S., et al. Balancing selection shapes density-dependent foraging behaviour. Nature. 539 (7628), 254-258 (2016).
- Greene, J. S., Dobosiewicz, M., Butcher, R. A., McGrath, P. T., Bargmann, C. I. Regulatory changes in two chemoreceptor genes contribute to a Caenorhabditis elegans QTL for foraging behavior. Elife. 5, (2016).
- Kim, K., et al. Two Chemoreceptors Mediate Developmental Effects of Dauer Pheromone in C. elegans. Science. 326 (5955), 994-998 (2009).
- McGrath, P. T., et al. Parallel evolution of domesticated Caenorhabditis species targets pheromone receptor genes. Nature. 477 (7364), 321-325 (2011).
- Schmitt, C., Schultheis, C., Husson, S. J., Liewald, J. F., Gottschalk, A. Specific Expression of Channelrhodopsin-2 in Single Neurons of Caenorhabditis elegans. PLoS ONE. 7 (8), e43164 (2012).
- White, J. G., Southgate, E., Thomson, J. N., Brenner, S. The Structure of the Nervous System of the Nematode Caenorhabditis elegans. Phil Trans of the Royal Soc of Lon. 314 (1165), 1 (1986).
- White, J. Q., et al. The sensory circuitry for sexual attraction in C. elegans males. Curr Biol. 17 (21), 1847-1857 (2007).
- Chronis, N., Zimmer, M., Bargmann, C. I. Microfluidics for in vivo imaging of neuronal and behavioral activity in Caenorhabditis elegans. Nat Meth. 4 (9), 727-731 (2007).
- Chute, C. D., Srinivasan, J. Chemical mating cues in C. elegans. Semin Cell Dev Biol. 33, 18-24 (2014).
- Izrayelit, Y., et al. Targeted metabolomics reveals a male pheromone and sex-specific ascaroside biosynthesis in Caenorhabditis elegans. ACS Chem Biol. 7 (8), 1321-1325 (2012).
- Ludewig, A. H., Schroeder, F. C. Ascaroside signaling in C. elegans. WormBook. , 1-22 (2013).
- Narayan, A., et al. Contrasting responses within a single neuron class enable sex-specific attraction in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 113 (10), E1392-E1401 (2016).
- Srinivasan, J., et al. A blend of small molecules regulates both mating and development in Caenorhabditis elegans. Nature. 454 (7208), 1115-1118 (2008).
- Sammut, M., et al. Glia-derived neurons are required for sex-specific learning in C. elegans. Nature. 526 (7573), 385-390 (2015).
- Sulston, J. E., Albertson, D. G., Thomson, J. N. The Caenorhabditis elegans male: postembryonic development of nongonadal structures. Dev Biol. 78 (2), 542-576 (1980).
- Hilliard, M. A., et al. In vivo imaging of C. elegans ASH neurons: cellular response and adaptation to chemical repellents. The EMBO Journal. 24 (1), 63-72 (2005).
- Evans, T. C. Transformation and microinjection. WormBook. , (2006).
- Cáceres, I. d. C., Valmas, N., Hilliard, M. A., Lu, H. Laterally Orienting C. elegans Using Geometry at Microscale for High-Throughput Visual Screens in Neurodegeneration and Neuronal Development Studies. PLoS ONE. 7 (4), e35037 (2012).
- Schrodel, T., Prevedel, R., Aumayr, K., Zimmer, M., Vaziri, A. Brain-wide 3D imaging of neuronal activity in Caenorhabditis elegans with sculpted light. Nat Methods. 10 (10), 1013-1020 (2013).
- García, L. R., Portman, D. S. Neural circuits for sexually dimorphic and sexually divergent behaviors in Caenorhabditis elegans. Curr Opin Neurobiol. 38, 46-52 (2016).
- Clokey, G. V., Jacobson, L. A. The autofluorescent "lipofuscin granules" in the intestinal cells of Caenorhabditis elegans are secondary lysosomes. Mech Ageing Dev. 35 (1), 79-94 (1986).
- Coburn, C., et al. Anthranilate Fluorescence Marks a Calcium-Propagated Necrotic Wave That Promotes Organismal Death in C. elegans. PLoS Biology. 11 (7), e1001613 (2013).
- Macosko, E. Z., et al. A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature. 458 (7242), 1171-1175 (2009).
- Park, D., et al. Interaction of structure-specific and promiscuous G-protein-coupled receptors mediates small-molecule signaling in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 109 (25), 9917-9922 (2012).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved