A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
A Facile Synthetic Method to Obtain Bismuth Oxyiodide Microspheres Highly Functional for the Photocatalytic Processes of Water Depuration
In This Article
Summary
This article describes a synthetic method to obtain bismuth oxyiodide microspheres, which are highly functional to perform the photocatalytic removal of organic pollutants, such as ciprofloxacin, in water under UV-A/visible light irradiation.
Abstract
Bismuth oxyhalide (BiOI) is a promising material for sunlight-driven-environmental photocatalysis. Given that the physical structure of this kind of materials is highly related to its photocatalytic performance, it is necessary to standardize the synthetic methods in order to obtain the most functional architectures and, thus, the highest photocatalytic efficiency. Here, we report a reliable route to obtain BiOI microspheres via the solvothermal process, using Bi(NO3)3 and potassium iodide (KI) as precursors, and ethylene glycol as a template. The synthesis is standardized in a 150 mL autoclave, at 126 °C for 18 h. This results in 2-3 µm-sized mesoporous microspheres, with a relevant specific surface area (61.3 m2/g). Shortening the reaction times in the synthesis results in amorphous structures, while higher temperatures lead to a slight increase in the porosity of the microspheres, with no effect in the photocatalytic performance. The materials are photo-active under UV-A/visible light irradiation for the degradation of the antibiotic ciprofloxacin in water. This method has demonstrated to be effective in interlaboratory tests, obtaining similar BiOI microspheres in Mexican and Chilean research groups.
Introduction
A plethora of semiconductors has been synthesized so far, aiming for photocatalysts with high activity under visible light irradiation, either to degrade organic compounds or to generate renewable energy in the form of hydrogen1,2. Bismuth oxyhalides BiOX (X = Cl, Br, or I) are candidates for such applications because of their high photocatalytic efficiency under visible light or simulated sunlight irradiation3,4. The band gap energy (Eg) of bismuth oxyhalides decreases with the increase of the atomic number of the halide; thus, BiOI is the material displaying the lowest activation energy (Eg = 1.8 eV)5. Iodide atoms, bonded via Van der Waals force to bismuth atoms, create an electric field that favors the migration of the charge carriers to the semiconductor surface, triggering the photocatalytic process4,6. Moreover, the architecture of the crystallite has a critical role in the separa,tion of the charge carriers. Highly oriented structures in the (001) plane and 3D structures (such as microspheres) facilitate the charge carrier separation upon irradiation, increasing the photocatalytic performance7,8,9,10,11,12. In light of this, it is necessary to develop reliable synthetic methods to obtain structures that boost the photo-activity of the bismuth oxyhalide materials.
The solvothermal method is, by far, the most commonly used and studied route to obtain BiOI microspheres13,14,15,16. Some methodologies using ionic liquids have been also reported17, although the expenses associated with these methodologies can be higher. Microsphere structure is usually obtained using organic solvents such as ethylene glycol, which acts as a coordinating agent to form metallic alkoxides, resulting in a gradual self-assembling of [Bi2O2]2+ species18,19. Using the solvothermal route with ethylene glycol facilitates the formation of different morphologies by changing the key parameters in the reaction, such as temperature and reaction time4,18. There is a wide body of literature on synthetic methods to obtain BiOI microspheres, which shows contrasting information to achieve highly photoactive structures. This detailed protocol is aimed at showing a reliable synthetic method to obtain BiOI microspheres highly functional in the photocatalytic degradation of pollutants in water. We intend to help new researchers to successfully obtain this kind of materials, avoiding the most common pitfalls associated with the synthesis process.
Access restricted. Please log in or start a trial to view this content.
Protocol
NOTE: Please read all the material safety data sheets (MSDS) before using the chemical reagents. Follow all the safety protocols by wearing a lab coat and gloves. Wear UV protection safety glasses during the photocatalysis tests. Be aware that nanomaterials may present important hazardous effects compared to their precursors.
1. Preparation of the BiOI microspheres
- For Solution 1, dissolve 2.9104 g of bismuth nitrate pentahydrate (Bi(NO3)3∙5H2O) in 60 mL of ethylene glycol in a glass beaker. For Solution 2, dissolve 0.9960 g of KI in 60 mL of ethylene glycol in a glass beaker.
NOTE: It is important to completely dissolve the inorganic salts in organic solvent; it may take around 60 min. Sonication may be helpful to dissolve both precursors. - Dropwise, add Solution 2 to Solution 1 (at a flow rate of approximately 1 mL/min). The colorless Solution 2 will change to a yellowish suspension. Sometimes, when Solution 2 is abruptly added, a black color may appear, due to the formation of the BiI4- complex. In such cases, the synthesis must be aborted and started again.
NOTE: Laboratory material must be completely dried since the occurrence of water promotes the uncontrolled precipitation of bismuth oxide (Bi2O3). - Stir the mixture, using a moderate speed for 30 min at room temperature. Then, transfer the mixture to a 150 mL autoclave reactor. Carefully swirl the beaker to remove the remaining suspension from the sidewalls. It is possible to add 1 to 5 mL of ethylene glycol to rinse the beakers. Make sure to tightly close the reactor.
NOTE: The autoclave should be filled from 40% to 80% of its capacity in order to achieve the optimal pressure conditions to the formation of the BiOI microspheres. A soft seal of the reactor may result in the loss of pressure, spoiling the synthesis. - Supply thermal treatment to the reactor in a furnace, from room temperature to 126 °C, using a temperature ramp of 2 °C/min. Maintain the final temperature for 18 h10. Then, cool the autoclave reactor to room temperature.
NOTE: Do not preheat the oven or provide a rapid heating since it will spoil the formation of the microspheres.
CAUTION: Do not induce cooling by washing the autoclave with cold water, as it may cause the deformation of the autoclave. Do not attempt to open the reactor while it is still hot, as this may result in the release of iodine gas.
2. Washing the BiOI microspheres
- Separate the solid material by decantation and wash it to remove ethylene glycol as much as possible. Prepare a filtration system consisting of a 0.8 μm filter paper (Grade 5, free of ashes) properly adhered to the walls of a glass funnel. Connect to an Erlenmeyer flask using a pierced cork stopper. Carry out the filtration step by gravity.
- (Optional) When pouring the suspension from the reactor to the funnel, use deionized water to rinse the autoclave reactor.
- Wash the solid product retained in the filter paper—of an intense orange color—several times with distilled water and absolute ethanol (technical grade). Alternate the washing solvent until the leachate is colorless.
NOTE: Please note that deionized water removes inorganic ions, while absolute ethanol removes the remaining ethylene glycol; thus, both solvents must be used. - Use deionized water in the two last washing steps to remove any trace of absolute ethanol and dry the intense-orange-colored product at 80 °C for 24 h. Last, store the material in amber glass bottles, in the dark, preferably in a desiccator.
3. Characterization of the BiOI microspheres
- Perform the X-ray diffraction analysis of the powder material, using a monochromic Cu-Kα light source, with λ = 1.5406 Å, operated at 30 kV and 15 mA.
- Determine the specific surface area by the Brunauer–Emmett–Teller (BET) method, via the adsorption of N2.
- Outgas the powder samples (500 mg) at 80ºC overnight prior to analysis. Perform the N2 adsorption measurements at -75ºC. Calculate the specific surface area and the pore volume from the adsorption isotherms.
- Determine the UV-visible diffuse reflectance spectra of the materials using a spectrophotometer with a praying mantis accessory.
- Dry the powder samples, in a laboratory oven, at 105ºC overnight. Then, carefully put 30 mg in the sample port of the praying mantis accessory.
- Irradiate the powder samples with a light source within the range of 200 to 800 nm in order to obtain the light absorption spectrum of the material. Calculate the band gap energy (Eg) using the absorption spectrum of the sample.
- Determine the secondary size of the BiOI microspheres by scanning electron microscopy.
- Put the powder sample on a carbon tape and then in the microscope stub to perform the observations.
- Determine the chemical composition of the samples by energy dispersive X-ray spectroscopy (EDS) analysis.
4. Photocatalytic activity test
- For the test solution, dissolve 7.5 mg of ciprofloxacin in 250 mL of distilled water, to obtain a 30 ppm solution. Then, transfer the test solution to the glass photocatalytic reactor. Thoroughly stir the solution, with a magnetic stirrer, keeping the temperature at 25 °C. Bubble air to the solution at 100 mL/min in order to maintain air saturation.
- Add 62.5 mg of the BiOI photocatalyst to the test solution to achieve a concentration of 0.25 g/L. Immediately, take the first sample (8 mL) using a glass syringe. After 30 min of stirring in the dark, take the second sample and turn the light source on.
- Given that the experiments are performed under UV-A/visible light conditions, use a 70 W lamp in the photocatalysis tests. Locate the light source 5 cm above the photoreactor.
- Take liquid samples (8 mL) after 5, 10, 15, 20, 30, 45, 60, 90, 120, 180, 240, and 300 min of irradiation. Filter all the withdrawn samples by passing them through a 0.22 µm nylon membrane, in order to remove any solid particle from the liquid prior to analysis. Store the filtered samples in amber glass vials at 4 °C until analysis.
- Determine the mineralization of ciprofloxacin by analyzing the total organic carbon (TOC) concentration remaining in the liquid samples throughout the photocatalytic process.
- Measure the concentration of total carbon (TC, in mg/L) via wet combustion at 720 °C, in the presence of a Pt catalyst and air atmosphere. Under such conditions, all the carbon is oxidized to CO2 and quantified in an FTIR detector coupled to the TOC device.
- Determine the inorganic carbon concentration (IC, in mg/L) via acidification of samples with 1 M HCl, leading to the conversion of carbonate and bicarbonate to CO2·H2O, which is quantified in the FTIR detector.
- Calculate the concentration of TOC remaining in water samples by the following equation.
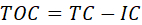
NOTE: In order to avoid interferences and, thus, incorrect results, it is very important to remove any trace of organic impurities by thoroughly cleaning all the glass material used in the sample preparation. This may be warranted by washing several times with hot water. - Calculate the mineralization yield via the depletion of total organic carbon throughout the reaction using the equation:
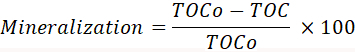
Here, TOCo is the concentration of total organic carbon at the beginning of the irradiation, while TOC is the concentration of total organic carbon at any time of the photocatalytic reaction.
Access restricted. Please log in or start a trial to view this content.
Results
3D microstructures of BiOI were successfully synthesized by the proposed synthetic method. This was confirmed by the SEM images shown in Figure 1a-c. The microspheres are formed from laminar structures of [Bi2O2]2+, which are bonded by two iodide atoms1. The formation of the microspheres depends on the temperature and time of the solvothermal procedure, as these paramet...
Access restricted. Please log in or start a trial to view this content.
Discussion
We consider the mixture of the precursors as the critical step in the solvothermal synthesis of the BiOI microspheres. A very slow dripping of the KI solution into the Bi(NO3)3 solution (at a maximum of 1 mL/min) is crucial to obtain mesoporous microspheres, since it allows the slow formation and self-assembly of the [Bi2O2]+2 slabs, followed by the bonding with the iodide atoms to form the BiOI laminates. The lamellae are the bricks of the microspheres in the solvot...
Access restricted. Please log in or start a trial to view this content.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors want to thank the Secretaría de Ciencia, Tecnología e Innovación de la Ciudad de México for the resources provided to carry out this work through the funded project SECITI/047/2016, and the National Funds for Scientific and Technological Development Chile (FONDECYT 11170431).
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| Bismuth(III) nitrate pentahydrate | Sigma Aldrich | 383074 | ACS reagent, ≥98.0% |
| Potassium iodide | Sigma Aldrich | 746428 | ACS reagent, ≥98.0% |
| Ethylene glycol | Sigma Aldrich | 324558 | Anhydrous, 99.8% |
| Ethanol | Meyer | 5405 | Technical Grade, 96% |
| Ciprofloxacin | Sigma Aldrich | 17850 | HPLC, ≥98.0% |
| Cary 5000 UV-Vis-NIR spectrophotometer | Agilent | Used for the Band gap determination by the Tauc model. | |
| JSM-5600 Scanning Electron Microscope | JOEL | Used for the SEM images. | |
| Autosob-1 | Qantachrome Instruments | Used for the determination of surface area and pore diameter. | |
| TOC-L Total Organic Carbon Analyzer | Shimadzu | Used for determination of total organic carbon in water samples. | |
| Bruker AXS D8 Advance - X-ray Diffraction | Bruker | Determination of crystal structure and crystallite size |
References
- Yu, C., Zhou, W., Liu, H., Liu, Y., Dionysiou, D. D. Design and fabrication of microsphere photocatalysts for environmental purification and energy conversion. Chemical Engineering Journal. 287, 117-129 (2016).
- Wang, H., et al. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chemical Society Reviews. 43 (15), 5234-5244 (2014).
- Chou, S. Y., Chen, C. C., Dai, Y. M., Lin, J. H., Lee, W. W. Novel synthesis of bismuth oxyiodide/graphitic carbon nitride nanocomposites with enhanced visible-light photocatalytic activity. RSC Advances. 6, 33478-33491 (2016).
- Siao, C. W., et al. Controlled hydrothermal synthesis of bismuth oxychloride/bismuth oxybromide/bismuth oxyiodide composites exhibiting visible-light photocatalytic degradation of 2-hydroxybenzoic acid and crystal violet. Journal of Colloid and Interface Science. 526, 322-336 (2018).
- Meng, X., Zhang, Z. Bismuth-based photocatalytic semiconductors: Introduction, challenges and possible approaches. Journal of Molecular Catalysis A: Chemical. 423, 533-549 (2016).
- Wang, Y., Deng, K., Zhang, L. Visible light photocatalysis of BiOI and its photocatalytic activity enhancement by in situ ionic liquid modification. Journal of Physical Chemistry C. 115 (29), 14300-14308 (2011).
- Xiao, X., Zhang, W. De Facile synthesis of nanostructured BiOI microspheres with high visible light-induced photocatalytic activity. Journal of Materials Chemistry. 20 (28), 5866-5870 (2010).
- Chen, C. C., et al. Bismuth oxyfluoride/bismuth oxyiodide nanocomposites enhance visible-light-driven photocatalytic activity. Journal of Colloid and Interface Science. 532, 375-386 (2018).
- Xia, J., et al. Self-assembly and enhanced photocatalytic properties of BiOI hollow microspheres via a reactable ionic liquid. Langmuir. 27 (3), 1200-1206 (2011).
- Mera, A. C., Contreras, D., Escalona, N., Mansilla, H. D. BiOI microspheres for photocatalytic degradation of gallic acid. Journal of Photochemistry and Photobiology A: Chemistry. 318, 71-76 (2016).
- Pan, M., Zhang, H., Gao, G., Liu, L., Chen, W. Facet-dependent catalytic activity of nanosheet-assembled bismuth oxyiodide microspheres in degradation of bisphenol A. Environmental Science and Technology. 49 (10), 6240-6248 (2015).
- Hu, J., et al. Solvents mediated-synthesis of BiOI photocatalysts with tunable morphologies and their visible-light driven photocatalytic performances in removing of arsenic from water. Journal of Hazardous Materials. 264, 293-302 (2014).
- Ye, L., Su, Y., Jin, X., Xie, H., Zhang, C. Recent advances in BiOX (X = Cl, Br and I) photocatalysts: Synthesis, modification, facet effects and mechanisms. Environmental Science: Nano. 1 (2), 90-112 (2014).
- Qin, X., et al. Three dimensional BiOX (X=Cl, Br and I) hierarchical architectures: Facile ionic liquid-assisted solvothermal synthesis and photocatalysis towards organic dye degradation. Materials Letters. 100, 285-288 (2013).
- Chou, S. Y., et al. A series of BiO x I y/GO photocatalysts: synthesis, characterization, activity, and mechanism. RSC Advances. 6 (86), 82743-82758 (2016).
- Shi, X., Chen, X., Chen, X., Zhou, S., Lou, S. Solvothermal synthesis of BiOI hierarchical spheres with homogeneous sizes and their high photocatalytic performance. Materials Letters. 68, 296-299 (2012).
- Di, J., et al. Reactable ionic liquid-assisted rapid synthesis of BiOI hollow microspheres at room temperature with enhanced photocatalytic activity. Journal of Materials Chemistry A. 2 (38), 15864-15874 (2014).
- Ren, K., et al. Controllable synthesis of hollow/flower-like BiOI microspheres and highly efficient adsorption and photocatalytic activity. CrystEngComm. 14 (13), 4384-4390 (2012).
- Lei, Y., et al. Room temperature, template-free synthesis of BiOI hierarchical structures: Visible-light photocatalytic and electrochemical hydrogen storage properties. Dalton Transactions. 39 (13), 3273-3278 (2010).
- Montoya-Zamora, J. M., Martínez-de la Cruz, A., López Cuéllar, E. Enhanced photocatalytic activity of BiOI synthesized in presence of EDTA. Journal of the Taiwan Institute of Chemical Engineers. 75, 307-316 (2017).
- He, R., Zhang, J., Yu, J., Cao, S. Room-temperature synthesis of BiOI with tailorable (0 0 1) facets and enhanced photocatalytic activity. Journal of Colloid and Interface Science. 478, 201-208 (2016).
- Song, J. M., Mao, C. J., Niu, H. L., Shen, Y. H., Zhang, S. Y. Hierarchical structured bismuth oxychlorides: self-assembly from nanoplates to nanoflowers via a solvothermal route and their photocatalytic properties. CrystEngComm. 12, 3875-3881 (2010).
- Mera, A. C., Váldes, H., Jamett, F. J., Meléndrez, M. F. BiOBr microspheres for photocatalytic degradation of an anionic dye. Solid State Science. 65, 15-21 (2017).
- Kong, X. Y., Lee, W. C., Ong, W. J., Chai, S. P., Mohamed, A. R. Oxygen-deficient BiOBr as a highly stable photocatalyst for efficient CO2 reduction into renewable carbon-neutral fuels. ChemCatChem. 8, 3074-3081 (2016).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved