Method Article
Postconditioning with Lactate-enriched Blood for Cardioprotection in ST-segment Elevation Myocardial Infarction
In This Article
Summary
Herein, we present a protocol for postconditioning with lactate-enriched blood, which is a novel approach for cardioprotection. This protocol comprises intermittent reperfusion and timely coronary injections of lactated Ringer's solution. This can be applied to ST-segment elevation myocardial infarction patients who undergo primary percutaneous coronary intervention.
Abstract
The beneficial effects of reperfusion therapy for ST-segment elevation myocardial infarction (STEMI) is attenuated by reperfusion injury. No approach has been proven successful in preventing this injury in the clinical setting to date. Meanwhile, a novel approach for cardioprotection in patients with STEMI, i.e., postconditioning with lactate-enriched blood (PCLeB), has recently been reported. PCLeB is a modification of the original protocol of postconditioning, aimed at increasing the delay in the recovery from tissue acidosis produced during ischemia. This was sought to achieve controlled reperfusion with tissue oxygenation and minimal lactate washout. In this modified postconditioning protocol, the duration of each brief reperfusion is gradually increased in a stepwise manner from 10 to 60 s. Each brief ischemic period lasts for 60 s. At the end of each brief reperfusion, injection of lactated Ringer's solution (20–30 mL) is performed directly into the culprit coronary artery immediately before the balloon inflation and the balloon is quickly inflated at the lesion site, so that the lactate is trapped inside the ischemic myocardium during each brief repetitive ischemic period. After seven cycles of balloon inflation and deflation, full reperfusion is performed. Stenting is performed thereafter, and the percutaneous coronary intervention is completed. Excellent in-hospital and 6 month outcomes in a limited number of patients with STEMI treated using PCLeB have already been reported. This method article provides a detailed description of each step of the PCLeB procedures.
Introduction
The widespread use of coronary reperfusion therapy has markedly improved the survival of patients with ST-segment elevation myocardial infarction (STEMI) over the past decades1,2. On the contrary, the proportion of patients with post-myocardial-infarction (post-MI) heart failure has increased, paradoxically3,4. To reduce the incidence of post-MI heart failure, further reduction of the infarct size is of paramount importance.
Timely restoration of the coronary blood flow is crucial for salvaging myocardial cells from the ischemic cell death. However, restoration of the coronary blood flow to the ischemic myocardium induces another type of cell death, which is myocardial reperfusion injury and reduces myocardial-salvaging effects of reperfusion therapy5,6. Therefore, to further reduce the infarct size and improve the outcomes of patients with STEMI, prevention of myocardial reperfusion injury is vital. However, despite notable efforts over the decades, no approach has been proven successful in preventing this injury in the clinical setting to date. Recently, a new approach for cardioprotection in patients with STEMI, i.e., postconditioning with lactate-enriched blood (PCLeB), has been reported7,8,9. PCLeB is a modification of the original protocol of postconditioning reported by Staat et al10. In the original postconditioning protocol, the culprit coronary artery is reopened and immediate application of four brief cycles of intermittent reperfusion is performed. Despite its initial success in a small pilot study10, the original postconditioning protocol has failed to improve the outcomes of patients with STEMI in large-scale clinical trials11,12,13. In the PCLeB protocol, the original postconditioning protocol was modified to increase the delay in recovery from tissue acidosis produced during ischemia because the delay in recovery from the tissue acidosis was thought to be responsible for the cardioprotective effects of postconditioning14. For this purpose, in addition to intermittent reperfusion, timely coronary injections of the lactated Ringer's solution were incorporated into the postconditioning protocol, aimed at achieving controlled reperfusion with tissue oxygenation and minimal lactate washout. Through this approach, the speed of the recovery from tissue acidosis relative to that of tissue re-oxygenation can be substantially reduced, thus making the delay in recovery from tissue acidosis definitive. The duration of each brief reperfusion, in this modified postconditioning protocol (Figure 1), is gradually increased in a stepwise manner from 10 to 60 s. This approach prevents the abrupt and rapid washout of lactate during the very early phase of reperfusion. Each brief ischemic period lasts for 60 s. Injection of lactated Ringer's solution is performed to supply lactate directly into the culprit coronary artery at the end of each brief reperfusion. 20 mL of lactated Ringer's solution is injected for the right coronary artery and 30 mL is injected for the left coronary artery. This is done immediately before the balloon inflation. During each brief repetitive ischemic period, to trap the lactate inside the ischemic myocardium, the balloon is quickly inflated at the lesion site. Full reperfusion is performed after seven cycles of balloon inflation and deflation. Stenting is performed thereafter, and the percutaneous coronary intervention (PCI) is completed. Excellent in-hospital15 and 6 month16 outcomes in a limited number of patients with STEMI treatment using PCLeB have already been reported. This method article provides a detailed description of each step of the PCLeB procedures, which is developed for preventing myocardial reperfusion injury in patients with STEMI.
Protocol
This protocol was approved by the ethics review board of the Saitama Municipal Hospital. The patient, whose case study is presented, provided written informed consent for the publication of his case and accompanying case images.
1. Set-up for PCLeB
- When starting coronary angiography (CAG), prepare the manifold for PCLeB. Connect one inlet line of the manifold to a contrast medium bottle and another inlet line to a 500 mL bottle of lactated Ringer’s solution mixed with 500 U of heparin sodium (Figure 2).
- Perform CAG and pinpoint the occluded lesion. After finishing CAG, introduce a guiding catheter, which is connected to the manifold via a Y-connector, into the aorta and engage it in the ostium of the culprit coronary artery as usual.
- Start wiring procedures with a balloon catheter placed inside the guiding catheter close to its outlet.
- If the coronary flow is incidentally restarted with the passage of the wire through the occluded lesion, move the balloon catheter quickly toward the culprit lesion to re-occlude the lesion site. After re-occluding the lesion site, resume and finish the wiring procedures.
NOTE: Such incidental coronary reflow sometimes happens; therefore, the balloon catheter is placed inside the guiding catheter during the wiring procedures. - After finishing the wiring procedures, move the balloon catheter forward and place the balloon at the occluded lesion. Check the position of the balloon by contrast medium injection, to see if the balloon is appropriately placed at the occluded lesion.
NOTE: After finishing the checking of the balloon position, naturally, the contrast medium fills all the lumen from the manifold to the tip of the guiding catheter. - Disconnect the syringe injector from the manifold and connect a 30 mL syringe to the manifold instead, which is used for lactated Ringer’s solution injection.
NOTE: No special syringe is needed for replacing the syringe, but an ordinary syringe for normal usage is fine as long as it has a ≥30 mL volume and a lock connector, so it will not be disconnected from the manifold during rapid injections of lactated Ringer’s solution. - Fill the 30 mL syringe with 20–30 mL of lactated Ringer’s solution by sucking it from the bottle of lactated Ringer’s solution through the line connected to the inlet of the manifold.
- Remove air bubbles inside the 30 mL syringe if present. Disconnect the syringe and push them out and re-connect it to the manifold. Suck lactated Ringer’s solution from the bottle to make up the amount used for pushing air bubbles out.
NOTE: Here, the default set-up of the injection system for PCLeB has been completed; that is, the contrast medium (approximately 4 mL) fills all the lumen from the manifold to the tip of the guiding catheter, and 20–30 mL of lactated Ringer’s solution fills the 30 mL syringe connected to the manifold.
2. Details of PCLeB Procedures
- Once the balloon catheter is placed at the occluded lesion and the default set-up of the injection system is completed, inflate the balloon for 20–30 s at low pressure.
- Deflate the balloon and start the initial 10 s brief reperfusion. Immediately push out all the contrast medium (approximately 4 mL) prefilled in the lumen from the manifold to the tip of the guiding catheter, by injecting ≥4 mL of 20–30 mL of the lactated Ringer’s solution prefilled in the 30 mL syringe into the guiding catheter, to see if the coronary flow is recovered.
NOTE: Approximately 4 mL of contrast medium is usually enough to confirm coronary flow recovery. - If the coronary flow is recovered, replenish the syringe with the same amount of lactated Ringer’s solution used for pushing the contrast medium out by sucking the solution from the bottle connected to the manifold through an inlet line, to prepare for lactated Ringer’s solution injection at the end of reperfusion. Jump to step 2.7.
NOTE: If coronary flow recovery is not observed, the true occluded lesion may be located distal to the current location of the balloon. Go to step 2.4. - Empty the syringe by injecting all the lactated Ringer’s solution into the coronary artery.
- Fill the syringe with 4 mL of the contrast medium by sucking it from the bottle connected to the manifold through an inlet line and inject it all to the guiding catheter to fill the lumen. Then, fill up the syringe with 20–30 mL of lactated Ringer’s solution by sucking it from the bottle of the lactated Ringer’s solution connected to the manifold through an inlet line.
NOTE: Here, the default set-up of the injection system for PCLeB has been re-established. - Move the balloon distally to the true lesion, and repeat the same procedures described in 2.1–2.6.
- Once coronary flow recovery is observed, and the balloon is confirmed to be placed at the culprit lesion, keep the balloon there throughout the PCLeB procedures.
NOTE: The desirable balloon size may be the same as the lesion diameter. By selecting such balloon size, instead of a smaller-sized balloon, the coronary flow may not be hampered by the deflated balloon left at the lesion site during each brief repetitive reperfusion. - Start lactated Ringer’s solution injection (20 mL for the right coronary artery and 30 mL for the left coronary artery) directly into the culprit coronary artery through the guiding catheter 4–5 s before the end of each brief reperfusion.
NOTE: Usually, it takes 5–7 s to inject 20–30 mL of lactated Ringer’s solution, which may overlap with the subsequent brief ischemic period; this is an intentional delay. The aim of this intentional delay is to ensure that the lactate is trapped inside the ischemic myocardium. - In the middle of lactated Ringer’s solution injection, let the secondary operator start balloon inflation so that the balloon inflation is completed a little before completion of lactated Ringer’s solution injection.
NOTE: This maneuver is intended so that the final small amount of lactated Ringer’s solution (ideally 2–3 mL) injected into the culprit coronary artery is bounced back from the inflated balloon. Through this approach, the lactated Ringer’s solution can be injected into the ischemic myocardium until the last moment of the balloon inflation process. This procedure requires cooperation between the primary operator who injects the lactated Ringer’s solution and the secondary operator who inflates the balloon. - Reestablish the default set-up of the injection system, as described in 2.5., during 60 s ischemia for the next brief reperfusion.
- Deflate the balloon and start the next 10 s longer reperfusion after 60 s ischemia. Push out all the contrast medium prefilled in the lumen from the manifold to the tip of the guiding catheter, to reconfirm coronary flow.
- If the balloon is found not to be placed at the center of the lesion site, adjust the balloon position.
NOTE: This might be done during the first 10 s reperfusion, but it can be postponed until the next 20 s reperfusion because the 10 s duration may be slightly very short to do it. - Start lactated Ringer’s solution injection into the culprit coronary artery through the guiding catheter 4–5 s before the end of each brief reperfusion
- In the middle of lactated Ringer’s solution injection, let the secondary operator start balloon inflation so that the balloon inflation is completed a little before completion of lactated Ringer’s solution injection.
- Reestablish the default set-up of the injection system during 60 s ischemia.
- Repeat these brief ischemia and reperfusion sequences seven times until 60 s reperfusion is performed twice (Figure 1).
NOTE: These procedures can be performed without disconnecting the 30 mL syringe from the manifold. The balloon inflation pressure can be increased gradually throughout the procedures, but high pressure is not recommended.
3. Alternative Default Set-up
NOTE: There may be an alternative to the default set-up, where replacing the syringe injector with a 30 mL syringe is not needed.
- After finishing the wiring procedures, disconnect the inlet line connected to the lactated Ringer’s solution bottle from the manifold and connect the 30 mL syringe filled with lactated Ringer’s solution directly with the inlet of the manifold instead.
NOTE: This is the alternative default set-up. - During each brief ischemia, disconnect the 30 mL syringe, which was emptied at the end of the preceding brief reperfusion, from the manifold. Refill the syringe with lactated Ringer’s solution by sucking it directly from the bottle. Reconnect the refilled 30 mL syringe to the inlet of the manifold to reestablish the alternative default set-up.
NOTE: With this set-up, it is possible to obtain more clear images of the culprit coronary artery by making a larger volume of contrast medium available. However, the aforementioned set-up may be more convenient because disconnecting the 30 mL syringe from the manifold is not necessary for each refilling of the syringe with lactated Ringer’s solution.
Results
A representative case treated using PCLeB is shown. A 48-year-old man presented at the emergency department of the Saitama Municipal Hospital for prolonged chest pain. Electrocardiography (ECG) revealed marked ST-segment elevation in precordial leads (Figure 3), and he was then diagnosed with anterior STEMI. Blood levels of glucose, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol were 111, 179, and 36 mg/dL, respectively. Glycated hemoglobin level was 5.9%. Creatine kinase (CK) and creatine kinase-MB (CK-MB) levels were 106 and 3 IU/L, respectively. The catheterization team was mobilized, and he was transferred to the catheterization laboratory immediately. He experienced frequent ventricular fibrillation before reperfusion therapy (Figure 4). DC cardioversion was performed three times at the emergency department and three times at the catheterization laboratory before CAG was performed.
CAG revealed proximal occlusion of the left anterior descending artery with thrombolysis-in-myocardial-infarction (TIMI) flow grade I (Figure 5A). No collateral circulation was observed. At this time, the aortic systolic pressure was approximately 70 mmHg (Figure 6A). However, intra-aortic balloon counterpulsation (IABP) was withheld because it enhances lactate washout via a mechanically driven force, which is opposite to what PCLeB does.
Reperfusion using PCLeB was started 60 min after symptom onset. No ventricular tachycardia/fibrillation was observed during and after PCLeB. The aortic systolic pressure increased to approximately 75 mmHg at the end of PCLeB (Figure 6B) and to 80 mmHg after the PCI (Figure 6C). Not only the systolic pressure but also the shape of the aortic pressure curve had improved; the apparent increase in the area under the curve of the aortic pressure suggested an increase in cardiac output. TIMI flow grade III was achieved after PCLeB. Stenting was performed, thereafter. TIMI flow grade III was still observed after stenting and the PCI was completed (Figure 5B). ECG recorded upon admission to the intensive care unit revealed complete ST resolution (Figure 7). The peak plasma CK and CK-MB levels were 4830 and 172 IU/L, respectively. The higher level of CK relative to that of CK-MB might be caused by a series of DC cardioversion before reperfusion therapy. No complication or adverse event was observed throughout his admission period. Resting thallium scintigraphy before discharge revealed well-preserved myocardial viability (Figure 8).
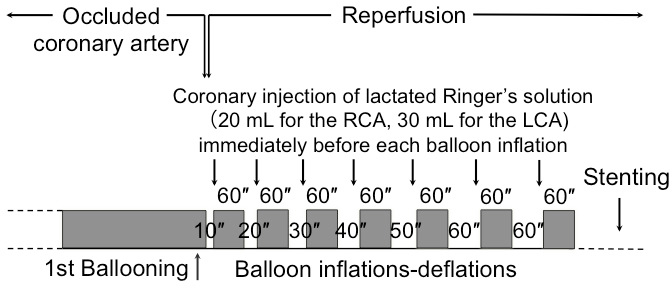
Figure 1: Overview of the protocol for postconditioning with lactate-enriched blood. The duration of each brief reperfusion is gradually increased from 10 to 60 s in a stepwise manner. Each brief ischemic period lasts for 60 s. At the end of each brief reperfusion, lactate is supplied by injecting lactated Ringer’s solution directly into the culprit coronary artery immediately before balloon inflation and the balloon is quickly inflated at the lesion site so that the lactate is trapped inside the ischemic myocardium. After seven cycles of balloon inflation and deflation, full reperfusion is performed, followed by stenting. LCA = left coronary artery; RCA = right coronary artery. This figure has been modified from reference8. Please click here to view a larger version of this figure.
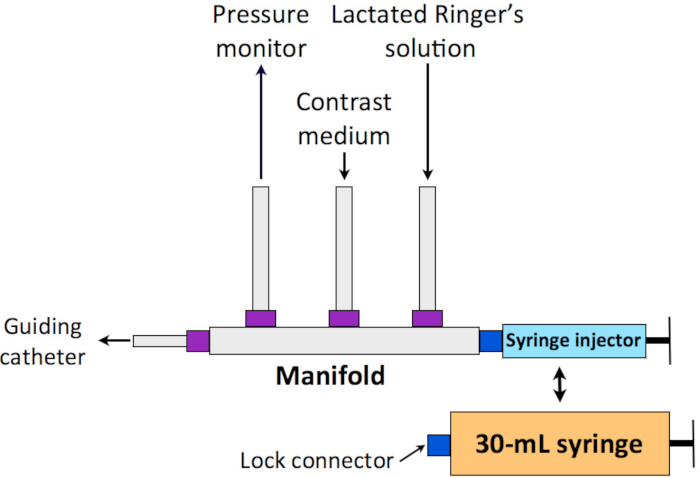
Figure 2: Schematic representation of the manifold system for PCLeB. One inlet line of the manifold is connected to a bottle of contrast medium and another inlet line to a 500 mL bottle of lactated Ringer’s solution. The syringe injector is to be replaced by a 30 mL syringe equipped with a lock connector before starting PCLeB. PCLeB = postconditioning with lactate-enriched blood. Please click here to view a larger version of this figure.
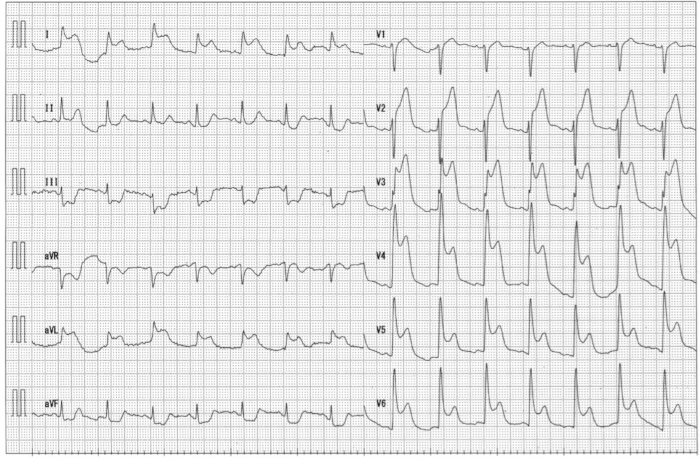
Figure 3: Electrocardiography in the emergency department. Marked elevation of ST-segment in precordial leads was observed. Please click here to view a larger version of this figure.
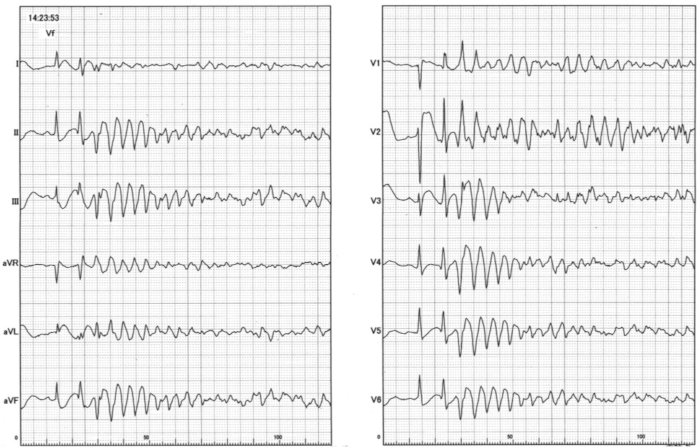
Figure 4: An electrocardiography recording of ventricular fibrillation observed at the catheterization laboratory Please click here to view a larger version of this figure.
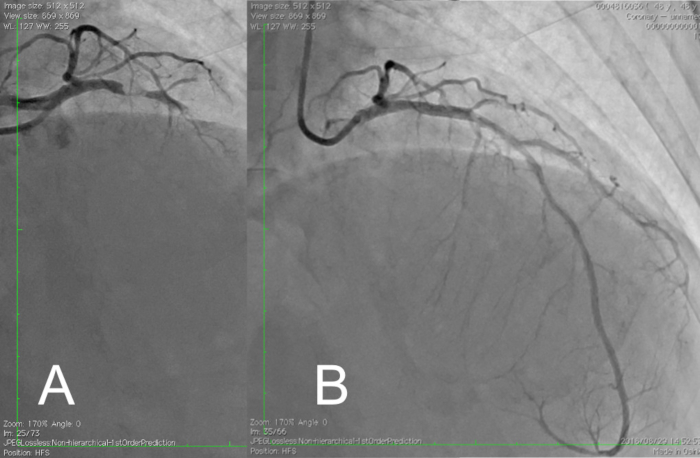
Figure 5: Coronary angiography view before and after reperfusion therapy with PCLeB. (A) Coronary angiography view before reperfusion therapy. Subtotal occlusion of the proximal left anterior descending artery with thrombolysis-in-myocardial-infarction flow grade I was observed. (B) Final coronary angiography view after reperfusion therapy with PCLeB. Coronary flow in the left anterior descending artery was resumed. PCLeB, postconditioning with lactate-enriched blood. Please click here to view a larger version of this figure.
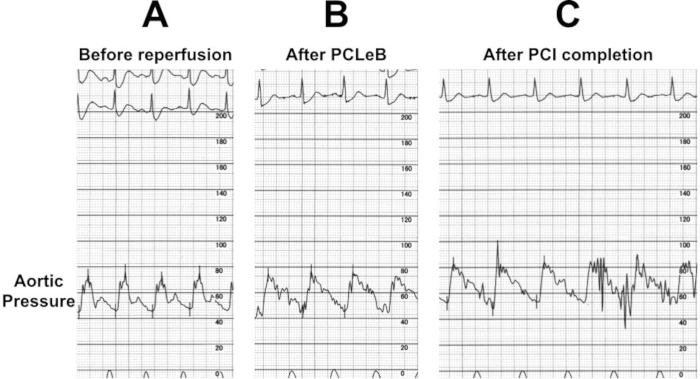
Figure 6: Aortic pressure before and after reperfusion with PCLeB. (A) Aortic pressure before reperfusion. The systolic aortic pressure was around 70 mmHg. (B) The aortic pressure immediately after PCLeB. The systolic aortic pressure increased to around 75 mmHg and the shape of the aortic pressure curve had improved in view of an increase in the area under the curve. (C) Aortic pressure after PCI completion. The systolic aortic pressure increased to 80 mmHg. PCI = percutaneous coronary intervention; PCLeB = postconditioning with lactate-enriched blood. Please click here to view a larger version of this figure.
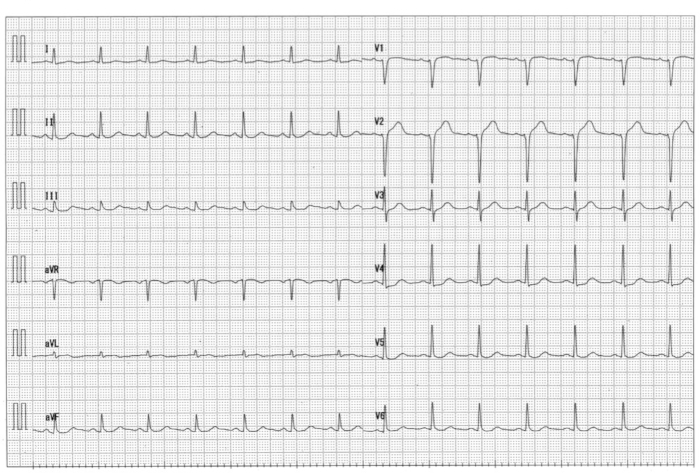
Figure 7: Electrocardiography at the intensive care unit immediately after reperfusion therapy Please click here to view a larger version of this figure.
Complete ST resolution was observed.
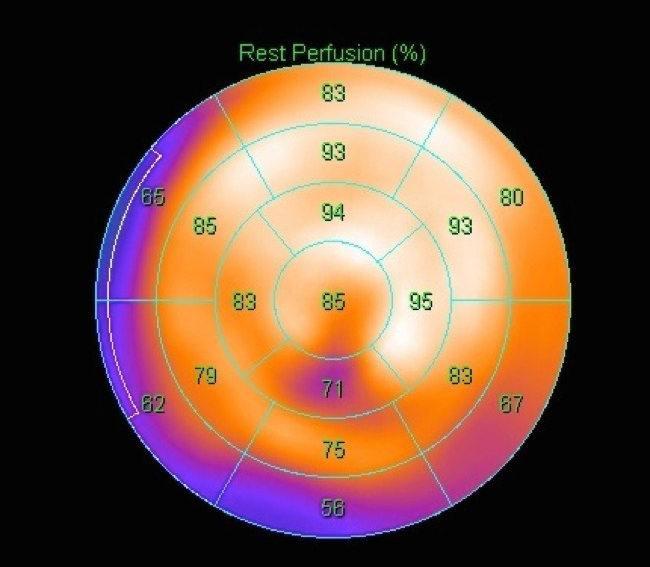
Figure 8: Resting thallium scintigraphy before discharge. A bull’s eye imaging is shown. Myocardial viability was well preserved in the left anterior descending artery area. Please click here to view a larger version of this figure.
Discussion
A detailed description of the PCLeB procedures was provided with a representative case treated using PCLeB. PCLeB consists of intermittent reperfusion and timely coronary injections of lactated Ringer's solution to achieve controlled reperfusion with tissue oxygenation and minimal lactate washout7,8,9. Not only intermittent reperfusion but also supplementary lactate, administered as lactated Ringer's solution, may increase the delay in recovery from tissue acidosis produced during ischemia; in this supposed mechanism, PCLeB may potentiate the beneficial effects of the original postconditioning protocol that consists of intermittent reperfusion only10.
Several critical steps need to be pointed out to successfully perform PCLeB. First, before starting PCLeB, incidental coronary flow recovery should be prevented as much as possible because uncontrolled reperfusion before PCLeB ruins the subsequent controlled reperfusion achieved by PCLeB in terms of tissue oxygenation with minimal lactate washout. Spontaneous reperfusion before CAG cannot help. However, during the initial wiring procedures, coronary flow is sometimes unintentionally re-started before balloon delivery to the occluded coronary artery. This is an unwanted phenomenon. To minimize the effects of this phenomenon, the balloon catheter is recommended to be placed inside the guiding catheter beforehand, close to its outlet during the wiring procedures, so that the balloon can be quickly moved forward to the culprit lesion to re-occlude the lesion site once the coronary flow is unintentionally re-started.
Second, once PCLeB is started, confirming the restoration of coronary flow during each brief reperfusion is important because the failure to restore the coronary flow during PCLeB procedures makes PCLeB meaningless. Therefore, during each brief reperfusion, contrast medium injection into the culprit coronary artery should be performed to check if the coronary flow was restored. This can be achieved by injecting ≥4 mL of 20–30 mL of the lactated Ringer's solution prefilled in the 30 mL syringe into the guiding catheter, which pushes out approximately 4 mL of the contrast medium prefilled within the lumen from the manifold to the tip of the guiding catheter in the default set-up. Injections of both the contrast medium and 20–30 mL of lactated Ringer' solution need to be performed even during the first 10 s reperfusion; thus, the busiest time during the entire procedures of PCLeB occurs at the very start of this protocol. Even if the initial brief reperfusion took 12–13 s instead of 10 s, it might be still acceptable. Since the reason for starting brief reperfusion with 10 s duration is to achieve minimal lactate washout during the very early phase of reperfusion, the initial brief reperfusion of 12–13 s duration can still achieve this goal.
Third, the size of the balloon used for PCLeB is important. Since the balloon is left at the lesion site throughout the procedures of PCLeB, if a small-sized balloon is selected, the lumen area gained after balloon dilation may be small and coronary flow may be hampered by the deflated balloon left at the lesion site during each brief reperfusion. Therefore, the balloon should ideally have the same size as the lumen diameter of the target lesion. However, one size smaller balloon may still be acceptable. Selection of balloons of such sizes is also beneficial in imposing adequate stretch stimuli on the vessel wall before stenting because of the reason mentioned later.
Fourth, the speed of lactated Ringer's solution injection and the timing of the balloon inflation are vital. The core objective of PCLeB is to keep tissue lactate concentrations high during the early reperfusion period. To achieve this objective, a larger amount of lactated Ringer's solution should be trapped inside the ischemic myocardium in a less diluted form. To make it possible, fast and continued injection of lactated Ringer's solution until the last moment of the balloon inflation process is needed. Therefore, 20–30 mL of lactated Ringer's solution needs to be injected within several seconds and the balloon inflation should be completed a little before completion of lactated Ringer's solution injection. To trap a larger amount of lactated Ringer's solution inside the ischemic myocardium, a larger amount of the solution, instead of 20–30 mL, can be used for each injection, but care should be taken to avoid volume overload.
Some modification of the PCLeB protocol might be possible if the modification adheres to the two critical components of PCLeB, i.e., starting with a very short period of reperfusion (i.e., 10–15 s) and trapping lactated Ringer's solution inside the ischemic myocardium in each brief repetitive ischemia. Reduction of the number of intermittent reperfusions and modification of the period of brief ischemia/reperfusion may be allowed. However, whether such modifications will reduce the beneficial effects of PCLeB is unknown.
Coronary flow recovery is generally very good after reperfusion therapy with PCLeB8,9,15. No-reflow phenomenon may not be experienced with this approach; this cannot be prevented by the original postconditioning protocol in animal experiments, reportedly17. However, at the end of the reperfusion therapy with PCLeB, if good coronary flow recovery cannot be achieved (i.e., TIMI grade flow II instead of III), there might be two possible explanations. First, insufficient stretch stimuli to the culprit lesion before stenting might reduce the beneficial effects of PCLeB. Stretch stimuli to the culprit lesion by balloon dilation or stenting procedures induces endothelin release from the endothelium of the lesion site18 and causes intracellular alkalization in myocardial cells19 distal to the lesion, which is an opposite effect of PCLeB20 and may reduce the beneficial effects of PCLeB. Therefore, it is recommended that supposed endothelin storage in the culprit lesion should be released as much as possible during the PCLeB procedures when tissue acidosis is maintained. If a smaller-sized balloon relative to the vessel size was used during the PCLeB procedures, stretch stimuli to the culprit lesion may be suboptimal and the endothelin inside the culprit lesion will be spared. Subsequent larger stimuli imposed by stenting procedures may induce an intense release of spared endothelin from the lesion site, possibly causing abrupt intracellular alkalization; this may attenuate the beneficial effects of PCLeB. Second, if a fairly longer stent, relative to the balloon used for PCLeB, is implanted, the similar phenomenon will ensue even if a sufficiently large balloon is used for the PCLeB procedures because no stretch stimuli is imposed on the coronary vessel wall excessively covered by the stent. Therefore, if possible, spot stenting using a shorter stent is preferable after the PCLeB procedures.
There might be no limitation in the application of PCLeB in patients with STEMI as long as reperfusion therapy is indicated. Cardiogenic shock is not a contraindication at all but rather a good indication for PCLeB. Increase in aortic pressure can be expected during PCI using PCLeB, as shown in the representative results. Simultaneous use of IABP may reduce the beneficial effects of PCLeB because IABP may enhance the washout of lactate via a mechanically driven force and may promote recovery from tissue acidosis produced during ischemia. Therefore, simultaneous use of IABP is not recommended even in severe cases, such as in patients with cardiogenic shock. Spontaneous reperfusion before CAG may reduce or remove the beneficial effects of PCLeB. However, spontaneous reperfusion before CAG does not necessarily preclude the application of PCLeB because coronary flow may still be insufficient and low-flow ischemia may still exist in the reperfused myocardium in such cases. Therefore, PCLeB may be worth trying as long as TIMI flow grade III is not achieved before PCI. Conversely, cases with spontaneous reperfusion with TIMI flow grade III achieved before PCI may be a clear limitation of the application of PCLeB.
The protocol of PCLeB appears complicated at a glance. However, once the initial busiest part of the protocol has finished, it can be realized that the patient's condition is becoming more stabilized as the procedures proceed to the less busy, later stages of the protocol. Before finishing the entire protocol, secondary operators often start to prepare for the next procedures after PCLeB, such as intravascular ultrasonography, because they know that nothing worse will happen, thereafter. Currently, myocardial reperfusion injury is generally left untreated during reperfusion therapy for STEMI. Despite the absence of firm evidence for the beneficial effects of PCLeB, considering its safety aspect and the current lack of alternative effective approaches, PCLeB is worth trying instead of leaving myocardial reperfusion injury as it occurs. Once the effectiveness of PCLeB is generally confirmed in the future, this technique might, hopefully, be applied to other arterial occlusive disorders, such as acute limb ischemia.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors have no acknowledgments.
Materials
| Name | Company | Catalog Number | Comments |
| Heparin Na (5000 U/5 mL) | Mochida Pharmaceutical Company | Heparin sodium | |
| Lactec Injection (500 mL) | Otsuka Pharmaceutical Factory | Lactated Ringer's solution | |
| NAMIC CONVENIENCE KIT AKK-3435 | NIPRO | 6069410 | a manifold-plus-syringe-injector kit |
| Y Connector | GOODMAN CO.,LTD. | YOL9A | Y-connector is connceted between a guiding catheter and a manifold, and enables both pressure momitoring and introduction of a balloon catheter into a guiding catheter simultaneously. |
References
- Nabel, E. G., Braunwald, E. A tale of coronary artery disease and myocardial infarction. New England Journal of Medicine. 366 (1), 54-63 (2012).
- Reed, G. W., Rossi, J. E., Cannon, C. P. Acute myocardial infarction. Lancet. 389 (10065), 197-210 (2017).
- Velagaleti, R. S., et al. Long-term trends in the incidence of heart failure after myocardial infarction. Circulation. 118 (20), 2057-2062 (2008).
- Ezekowitz, J. A., Kaul, P., Bakal, J. A., Armstrong, P. W., Welsh, R. C., McAlister, F. A. Declining in-hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. Journal of American College of Cardiology. 53 (1), 13-20 (2009).
- Piper, H. M., Garcia-Dorado, D., Ovize, M. A fresh look at reperfusion injury. Cardiovascular Research. 38 (2), 291-300 (1998).
- Yellon, D. M., Hausenloy, D. J. Myocardial reperfusion injury. New England Journal of Medicine. 357 (11), 1121-1135 (2007).
- Koyama, T., Shibata, M., Moritani, K. Ischemic postconditioning with lactate-enriched blood in patients with acute myocardial infarction. Cardiology. 125, 92-93 (2013).
- Koyama, T., et al. Impact of ischemic postconditioning with lactate-enriched blood on early inflammation after myocardial infarction. IJC Metabolic & Endocrine. 2, 30-34 (2014).
- Koyama, T. Lactated Ringer’s solution for preventing myocardial reperfusion injury. IJC Heart & Vasculature. 15, 1-8 (2017).
- Staat, P., et al. Postconditioning the human heart. Circulation. 112 (14), 2143-2148 (2005).
- Hahn, J. Y., et al. Ischemic postconditioning during primary percutaneous coronary intervention: the effects of postconditioning on myocardial reperfusion in patients with ST-segment elevation myocardial infarction (POST) randomized trial. Circulation. 128 (17), 1889-1896 (2013).
- Limalanathan, S., Andersen, G. &. #. 2. 1. 6. ;., Kløw, N. E., Abdelnoor, M., Hoffmann, P., Eritsland, J. Effect of ischemic postconditioning on infarct size in patients with ST-elevation myocardial infarction treated by primary PCI results of the POSTEMI (POstconditioning in ST-Elevation Myocardial Infarction) randomized trial. Journal of American Heart Association. 3 (2), e000679 (2014).
- Engstrøm, T., et al. Effect of ischemic postconditioning during primary percutaneous coronary intervention for patients with ST-segment elevation myocardial infarction: a randomized clinical trial. JAMA Cardiology. 2 (5), 490-497 (2017).
- Inserte, J., Barba, I., Hernando, V., Garcia-Dorado, D. Delayed recovery of intracellular acidosis during reperfusion prevents calpain activation and determines protection in postconditioned myocardium. Cardiovascular Research. 81 (1), 116-122 (2009).
- Koyama, T., et al. Impact of postconditioning with lactate-enriched blood on in-hospital outcomes of patients with ST-segment elevation myocardial infarction. International Journal of Cardiology. 220, 146-148 (2016).
- Koyama, T., Munakata, M., Akima, T., Miyamoto, K., Kanki, H., Ishikawa, S. Muscle squeezing immediately after coronary reperfusion therapy using postconditioning with lactate-enriched blood. International Journal of Cardiology. 275, 36-38 (2019).
- Hale, S. L., Mehra, A., Leeka, J., Kloner, R. A. Postconditioning fails to improve no reflow or alter infarct size in an open-chest rabbit model of myocardial ischemia-reperfusion. American Journal of Physiology-Heart and Circulatory Physiology. 294 (1), H421-H425 (2008).
- Hasdai, D., Holmes, D. R., Garratt, K. N., Edwards, W. D., Lerman, A. Mechanical pressure and stretch release endothelin-1 from human atherosclerotic coronary arteries in vivo. Circulation. 95 (2), 357-362 (1997).
- Kohmoto, O., Ikenouchi, H., Hirata, Y., Momomura, S., Serizawa, T., Barry, W. H. Variable effects of endothelin-1 on [Ca2+]i transients, pHi and contraction in ventricular myocytes. American Journal of Physiology-Heart and Circulatory Physiology. 265, H793-H800 (1993).
- Koyama, T. Postconditioning with lactate-enriched blood in patients with ST-segment elevation myocardial infarction. Cardiology. 142, 79-80 (2016).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved