Method Article
A Minimally Invasive Lesion Technique for Muscles Intrinsic to the Odontophore of Aplysia californica
In This Article
Summary
Here we present a protocol for minimally invasive surgical lesioning of muscles intrinsic to the feeding apparatus of the marine mollusk Aplysia californica to understand the roles of these muscles during feeding behavior.
Abstract
Aplysia californica is a model system for studying the neural control of learning and behavior. This animal has a semi-open circulatory system, making it possible to access many of its internal structures without causing any significant damage. Many manipulations can be easily performed both in vivo and in vitro, making it a highly tractable model for the analysis of behavior and neural circuitry. To better understand the functions of muscles within the feeding grasper, we have developed a technique for lesioning them without opening the main body cavity of the animal or damaging the outer layers of the feeding organ (i.e., the buccal mass). In this technique, the grasper is partially everted, allowing direct access to the musculature. This procedure allows animals to recover quickly and reliably. This has made it possible to lesion the I7 muscles and sub-radular fibers, allowing us to show that both muscles significantly contribute to the opening in vivo.
Introduction
The feeding system of Aplysia californica has a long history of use as a model system for understanding learning and memory1, motivated behaviors2,3, and the interaction between behavior, biomechanics and neural control during feeding4. It has highly accessible neural circuitry, with a relatively small number of large, identifiable neurons. The animal has a semi-open circulatory system, making it possible to access many of its internal structures without causing significant damage. It is also robust to many manipulations both in vivo and in vitro, making it a highly tractable model for the analysis of behavior and neural circuitry.
To understand the neural patterns that give rise to feeding behaviors, it is important to describe the underlying mechanics of the soft structure that makes up the feeding organ, the buccal mass4. While there has been work done to characterize the outer muscles that make up the buccal mass5,6, the inner muscles of the underlying structure within the buccal mass that controls the surface of the grasper, the odontophore, have been largely inaccessible to in vivo experimentation. Although there have been in vitro studies on the functions of some of these muscles7,8, the lack of direct access to these muscles has made it difficult to study their role in intact, behaving animals.
Most techniques for electrode implantation or lesions in Aplysia or similar molluscan species require that the body wall be opened9,10,11,12. Opening the body wall causes epithelial injury, and the incision must be securely sealed to prevent hemolymph escape. Even more serious difficulties are posed when attempting to reach the inner muscles of the grasper of Aplysia (muscles underlying the radular surface or within the odontophore): having entered through the main body cavity, one must then go through some portion of the muscular wall of the buccal mass to gain access to the interior structures (Figure 1A). This accumulated injury and difficulty of access has made the approach through conventional means problematic because animals do not recover well from these surgeries (of animals with full eversions, only 17% regained any feeding ability, N = 12. Around 85% of non-everted animals regained the ability to feed, N = 84).
The I7 muscle, which has been characterized as a radular opener8, is deep inside the odontophore itself, further complicating access. It stretches between the base of the radular stalk (Figure 1C) and the underside of the radular surface, through a lumen in the odontophore (Figure 1C). On three sides of the I7 muscles are walls of muscle, and the fourth wall consists of the radular stalk. For the purposes of a biomechanical study, major impairment to any of these structures would compromise the normal function of the feeding apparatus. We developed a novel approach of working the odontophore out through the jaws, and conducting the surgery through an incision to the thin, cartilaginous radular surface, that made it possible to lesion the I7 muscle, as well as newly-described fine muscle fibers that run just beneath the radular surface, which we refer to as the sub-radular fibers (Figure 1C).
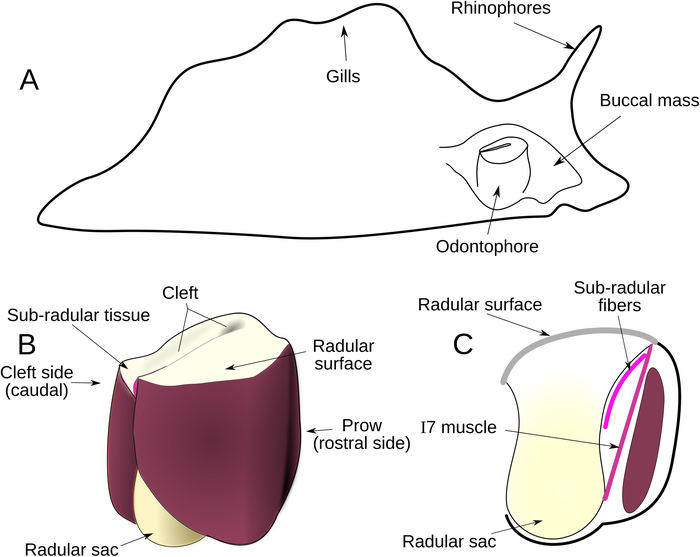
Figure 1: Anatomical Overview. (A) Location of the buccal mass within Aplysia. (B) External anatomy of odontophore. The surface of radula and radular sac are yellow; muscles composing the odontophore are shown in red, based on their actual colors. (C) Sagittal section of odontophore, showing the location of sub-radular fibers (curved pink line) and I7 muscle (straight pink line). Cross section of the I6 muscle is shown in dark red. Please click here to view a larger version of this figure.
Protocol
Aplysia are invertebrates and thus not subjected to IAUC approval. To minimize discomfort to animals, ensure that they are fully anesthetized before applying the surgical techniques described below.
1. Animal Selection and Anesthetization
- Select an active animal by offering it seaweed and confirming that bite intervals are no greater than between 3 and 5 s.
- Anesthetize the animal with 0.333 molar magnesium chloride (see Table 1) by injecting near the head with an 18 G needle on a 60 mL syringe so that the highest concentration of anesthetic will be around the buccal mass.
- Take care to penetrate both the outer epithelium and the inner tissue layer with the needle. Ensure that the injection is roughly under the rhinophore, halfway between the rhinophore and the foot, and the needle should enter obliquely, pointing in the direction of the jaws.
- After 10 min, gently attempt to insert a pin into the gill and rhinophore, verifying that these do not retract, to ensure sufficient anesthetization.
- Ensure that the lips and jaw of the slug are relaxed so that the odontophore can be exposed.
NOTE: The wrinkling on the lips of Figure 2A indicates that the animal’s lips and jaw are not sufficiently relaxed for the surgical procedure to be performed without damage. The smooth, relaxed lips of Figure 2B indicate that the jaws are fully relaxed.
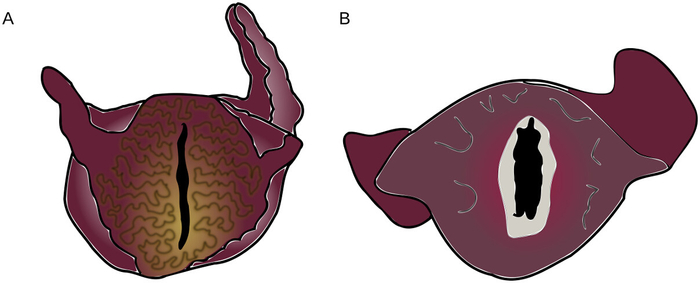
Figure 2: Tension and Relaxation in Anesthetized Aplysia Mouths. (A) Aplysia showing a high degree of muscle tension around the lips. This correlates with jaw tension and contraindicates proceeding with the surgery. (B) Aplysia with relaxed lips, showing the inside of jaws (light grey). Colors again correspond to those observed in the animal. Please click here to view a larger version of this figure.
- If an animal’s lips are not relaxed, inject an additional 30 mL of magnesium chloride and wait another 5 min. If this does not result in lip relaxation, return them to an isolated container with good water flow to allow them to recover (see step 4) and proceed with a different animal.
2. Exposing the Radular Surface
- Position the slug so that the head hangs downward, allowing the buccal mass to settle against the jaws.
- Apply pressure with the thumb and forefinger to push the buccal mass toward the jaws, holding the buccal mass in place.
- Rotate the jaws so that they are visible. At the same time, maintain the pressure on the buccal mass so that the prow of the buccal mass is visible through the jaws. (Figure 3).
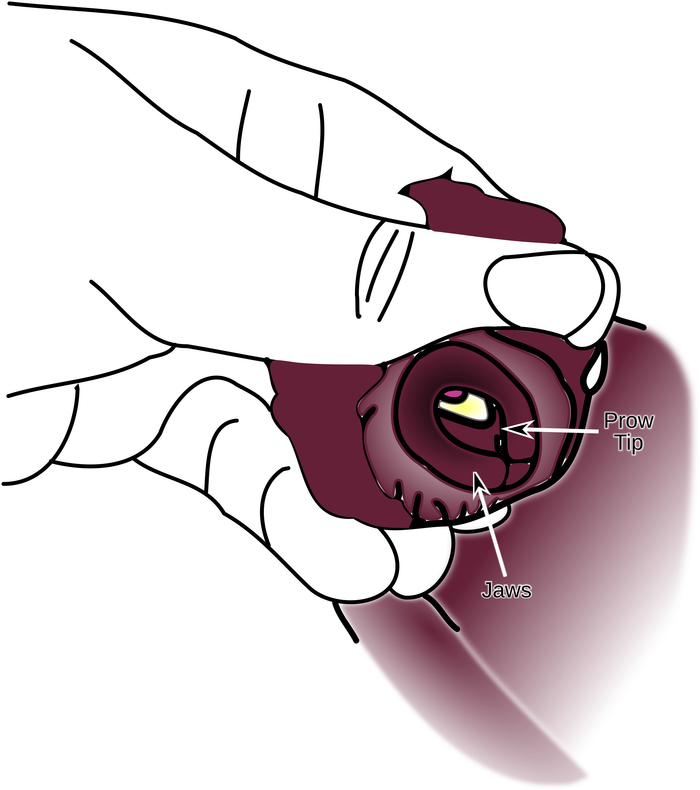
Figure 3: Supporting the Buccal Mass Against the Inside of the Jaws. Fingers support the buccal mass that has been pushed up against the inside edge of the jaws until the tip of the prow can be seen. Please click here to view a larger version of this figure.
- Gently work the tips of the blunt forceps into the cleft of the odontophore and use them to lever the radular surface through the jaws. If the jaws are not sufficiently relaxed, use the forceps to gently grasp the edge of the cleft to assist this process.
CAUTION: This pressure does risk greater damage to the animal. - Once the surface is exposed, work the jaws clear of the anterior portion of the radular surface all the way around the perimeter. This makes the odontophore less likely to retract (Figure 4). Ensure that no more than half of the walls of the odontophore is exposed.
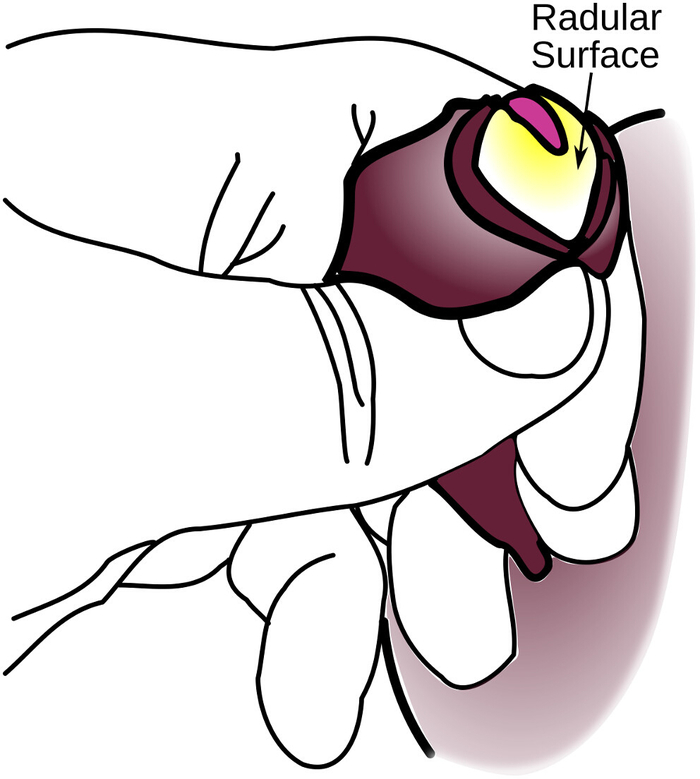
Figure 4: Partial Eversion of the Odontophore. The radular surface is fully exposed, but the sides of the odontophore are not uncovered, making this only a partial eversion. Further eversion will likely result in damage to the animal. Please click here to view a larger version of this figure.
NOTE: A full eversion of the odontophore will cause major muscle damage from which the animals are very slow to recover.
3. Surgical Incisions
- Once the radular surface is fully exposed, arrange the slug under a dissection scope for the surgery.
- Alternatively, use a wide rubber band and a third hand to stabilize the jaws and radular surface for the surgery, especially while learning. This, however, adds time and increased tissue damage to the procedure, which makes it less ideal over the long term.
- Place the radular surface so that the cleft side faces the investigator.
- Gently grasp the radular surface, near the radular base, so that a horizontal fold is formed perpendicular to the anatomical crease. Use fine scissors to cut through this fold, making an incision along the anatomical crease (Figure 5).
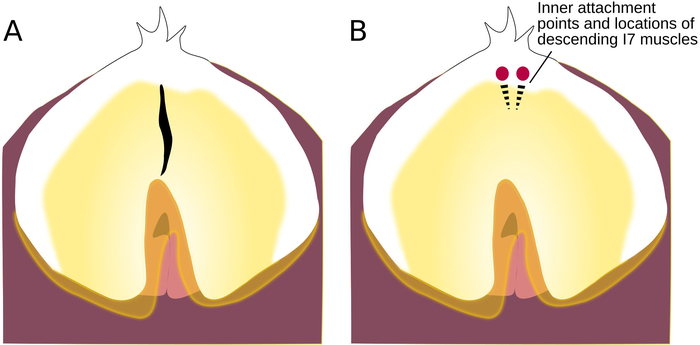
Figure 5: Location of Incision to the Radular Surface. (A) Radular surface, with an incision. (B) Radular surface with circles showing where the strands of the bilateral I7 muscle attach; dotted lines show the location of the descending muscles underneath the radular surface. Please click here to view a larger version of this figure.
- Extend this initial incision to 3-5 cm to allow access to the interior of the buccal mass.
- Adjust light so that it points directly back through this incision.
- Part the edges of the incision so that the back of the lumen of the odontophore and the thin vertical strands of the I7 muscle are visible. (Figure 6)
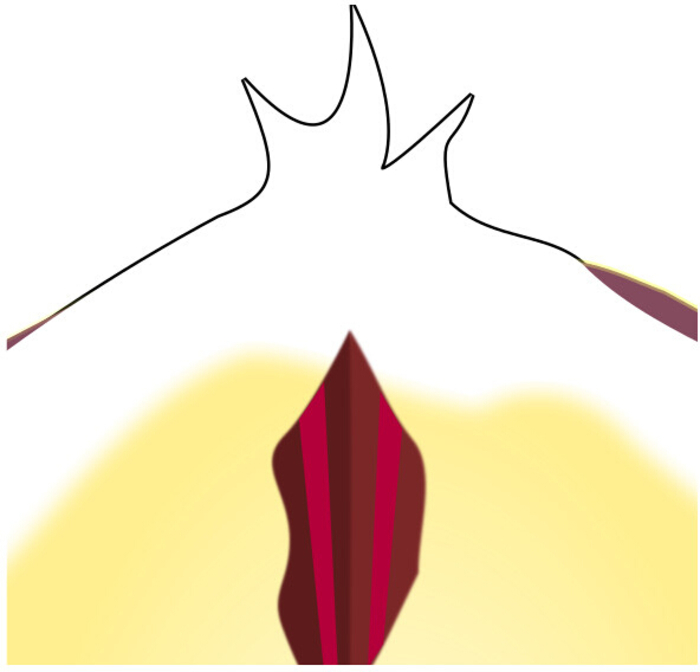
Figure 6: Location of I7 through the Radular Surface Incision. Looking through the incision, both strands of I7 can be seen between the inner surfaces of I4. Please click here to view a larger version of this figure.
- Reach into through the incision, grasp both strands of I7, and pull them up through the incision, where as much as the muscle can be cut away as is practical (Figure 7).
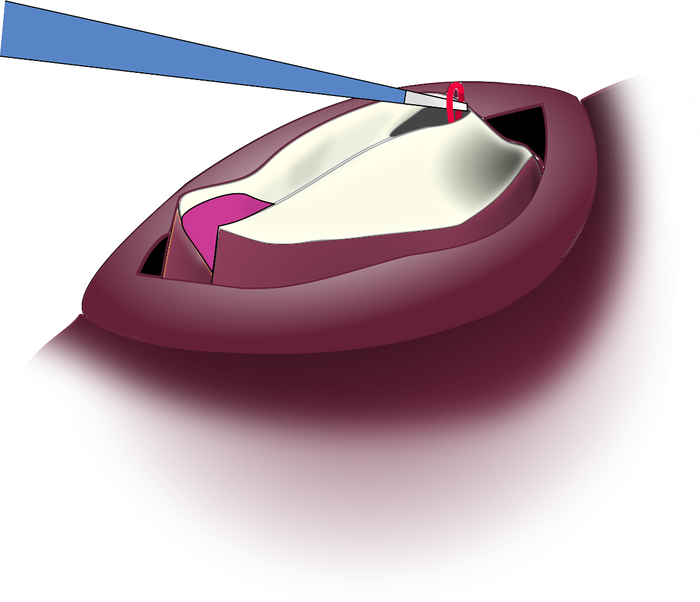
Figure 7: Pulling the I7 Muscle Strand Through the Incision. The I7 muscle is highly elastic and can be pulled up through the incision for removal. Please click here to view a larger version of this figure.
NOTE: With practice, it is usually more effective to locate I7 by feel than by sight.
4. Post-operative Care
- After lesions have been performed, grasp the anterior tentacles, and push down on the radular surface to return the slug to its original configuration.
- Place post-surgical animals in a protected environment with good water flow. Increased oxygenation speeds the recovery. Ensure that the animals are alert and responsive on the day after surgery. If this is not the case, it can be assumed that they will not recover.
NOTE: Animals will usually begin to feed on the first or second day after surgery. Even animals that are having trouble biting should be offered seaweed, as it is our anecdotal observation that an animal’s recovery is improved by its attempts to eat.
5. For Sub-radular Fiber Lesion
- Follow the steps from 1.1 through 3.5
- Insert the tip of a small straight scalpel blade (#11 or similar) through the incision with the sharp edge angled upwards. Gently scrape the fine muscular fibers from the underside of the radular surface. (Figure 8).
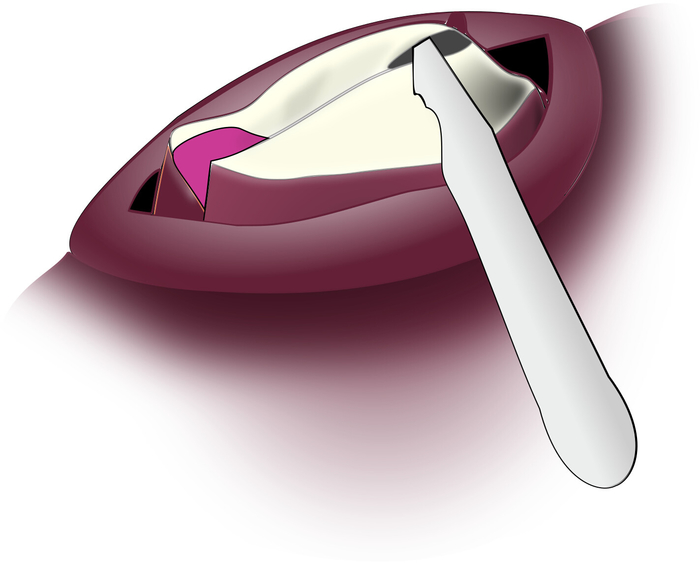
Figure 8: Lesioning the Subradular Fibers. The edge of the scalpel blade is angled upwards through the incision to the underside of the radular surface so that it can gently scrape away the sub-radular fibers. Please click here to view a larger version of this figure.
- Return to step 4.1.
Results
Previous work had suggested that the I7 muscle contributed to the opening of the grasper8. Our own anatomical studies suggested that the sub-radular fibers might also contribute to grasper opening. To test these hypotheses, animals were induced to generate bites both before and after receiving a surgical procedure. Sham animals were subjected to all the surgical steps, including the incision in the radular surface, but no internal muscles were removed. Animals subjected to an I7 lesion had both I7 muscles removed. Animals subjected to a sub-radular fiber lesion had ~25% of the sub-radular fibers removed immediately beneath the incision. Sham lesions had no significant effect on the width of the opening at the peak of biting, whereas both I7 and sub-radular fibers lesions did significantly reduce bite width (Figure 9).
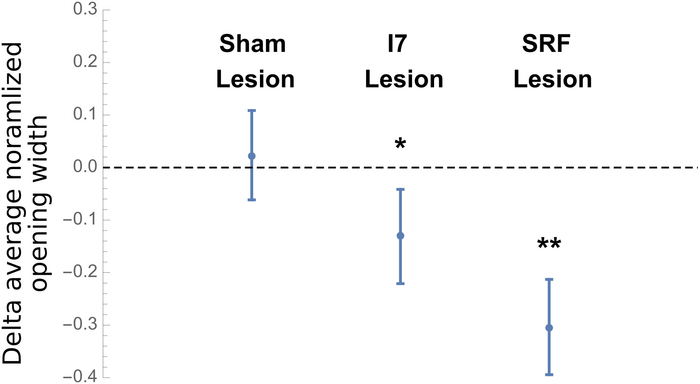
Figure 9: Results of Lesions on Opening Width During Peak Biting. Data shown are the differences between the averaged normalized opening width of the radula before and after the surgical procedure for 5 animals in each of the 3 groups (sham, I7 lesion, or SRF lesion), with each animal serving as its own control. Averages were taken of 5 bites before, and 5 bites after the surgical procedure to determine the mean normalized difference. Opening width was the distance from the center of radula to the radular edge at the peak protraction, normalized by the distance from the inner surface of the radular base to the cleft-side edges of the radular surface. The differences are shown as the means plus or minus the standard deviation. After establishing that the difference data were normally distributed, the probability that the lesion had no effect was determined (i.e., the null hypothesis was tested that the effects of the surgical procedures would be zero, on average) by applying a paired t-test to each independent group. The data demonstrates that the sham lesion had no significant effect, whereas a lesion of the I7 muscles or a lesion of the sub-radular fibers did have a significant effect on radular opening (p < 0.031 for the I7 lesion group, indicated with a single asterisk, or p < 0.002 for the SRF lesion group, indicated by a double asterisk). Please click here to view a larger version of this figure.
| Body Weight | Magnesium Chloride Dose |
| <200 g | ½ bodyweight |
| 200-350 g | 1/3 bodyweight |
| 350-450 g | ¼ bodyweight |
Table 1: Magnesium Chloride dosage by bodyweight.
Discussion
The most critical steps within the protocol are the need to ensure that the animal is fully anesthetized, and that the eversion of the buccal mass is just enough to access the underlying muscles. It may require some practice to perfect these steps, but once they are mastered, the yield from surgeries is likely to be greater than 85% of all experiments done. The most important way to properly modify and troubleshoot the protocol is to spend time doing dissections of the buccal mass so that the locations of the internal muscles are completely clear to the investigator. Because the suggested incision through the radular surface inevitably causes some damage to the underlying sub-radular fibers, it may be appropriate to modify the exact location of the incision to avoid specific regions of these fibers.
One limitation of the surgical technique is that it may have non-specific effects on feeding responses, such as the strength of protraction. One way to overcome this limitation is to have animals serve as their own controls. In addition, it is critical to have a sham lesion group which is subjected to the entire surgical protocol except for the removal of the specific muscle (i.e., I7 or the SRFs). By following these suggestions, an investigator will reduce the effects of variability between animals and have an intrinsic measure of the non-specific effects of surgery.
Previous work has used approaches through the body wall to lesion or record either from nerves13,14, or muscles15,16,17. In our laboratory, we have anecdotally observed that body wall incisions are often accompanied by a significant loss of hemolymph and thus of body volume. Animals often require several days to recover from this, and if the body wall lesion is not carefully sutured, animals may not recover. In addition, post-mortem examination of the animals reveals considerable scarring around the incision and a strong immune response (anecdotal observations). In contrast, animals show no loss of hemolymph or change in body volume after recovery from the protocol described here (based on observations in 96 animals).
Future applications of the technique may extend it to other muscles within the feeding apparatus of Aplysia, and to other animals. We have focused on the removal of the I7 muscle and sub-radular fibers. These same general surgical techniques also allow access to most of the other muscles of the odontophore. Some of these, such as the internal portion of the I5 muscle, are best accessed through the radular surface. Others, like the inner leaflets of I4, may be better reached through the exterior epithelium of the odontophore. We have made preliminary trials where an incision under the radular cleft of the partially everted odontophore allowed access for a sharpened hook to be inserted that could then be used to lesion another muscle within the odontophore, muscle I88. Because the surgical protocol described here does not open the main body cavity, no suturing is required.
The protocol that we have described may be of general interest to other investigators working on soft tissue structures that would otherwise be difficult to manipulate, e.g., the feeding apparatus of other mollusks. More generally, this protocol could suggest other novel surgical approaches to the analysis of soft structures such as tongues, trunks or tentacles18.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We would like to acknowledge the hard work that Sherry Niggel, Sisi Lu, and Joey Wu put into improving and validating these protocols. This work was supported by NSF Grant IOS 1754869.
Materials
| Name | Company | Catalog Number | Comments |
| Blunt forceps | Fine Science Tools | 11210-10 | 2 pair |
| Scalpel blade (#11) | Fine Science Tools | 10011-00 | |
| Spring scissors | Fine Science Tools | 15024-10 | |
| Webcam | Logitech | c920 | for recording data |
References
- Pinsker, H., Kupfermann, I., Castellucci, V., Kandel, E. Habituation and Dishabituation of the GM-Withdrawal Reflex in Aplysia. Science. 167, 1740-1742 (1970).
- Kupfermann, I. Feeding Behavior in Aplysia: A Simple System for the Study of Motivation. Behavioral Biology. 10, 1-26 (1974).
- Susswein, A. J., Chiel, H. J. Nitric oxide as a regulator of behavior: New ideas from Aplysia feeding. Progress in Neurobiology. 97, 304-317 (2012).
- Chiel, H. J. Aplysia feeding biomechanics. Scholarpedia. 2, 4165 (2007).
- Neustadter, D. M., Drushel, R. F., Chiel, H. J. Kinematics of the buccal mass during swallowing based on magnetic resonance imaging in intact, behaving Aplysia californica. Journal of Experimental Biology. 205, 939-958 (2002).
- Neustadter, D. M., Herman, R. L., Drushel, R. F., Chestek, D. W., Chiel, H. J. The kinematics of multifunctionality: comparisons of biting and swallowing in Aplysia californica. Journal of Experimental Biology. 210, 238-260 (2007).
- Brezina, V., Evans, C. G., Weiss, K. R. Characterization of the membrane ion currents of a model molluscan muscle, the accessory radula closer muscle of Aplysia california. I. Hyperpolarization-activated currents. Journal of Neurophysiology. 71, 2093-2112 (1994).
- Evans, C. G., Rosen, S., Kupfermann, I., Weiss, K. R., Cropper, E. C. Characterization of a Radula Opener Neuromuscular System in Aplysia. Journal of Neurophysiology. 76 (2), 1267-1281 (1996).
- Cullins, M. J., Chiel, H. J. Electrode Fabrication and Implantation in Aplysia californica for Multi-channel Neural and Muscular Recordings in Intact, Freely Behaving Animals. Journal of Visualized Experiment. (40), 1791 (2010).
- Dudek, F. E., Cobbs, J. S., Pinsker, H. M. Bag cell electrical activity underlying spontaneous egg laying in freely behaving Aplysia brasiliana. Journal of Neurophysiology. 42, 804-817 (1979).
- Hermann, P., Maat, A., Jansen, R. The Neural Control of Egg-Laying Behaviour in the Pond Snail Lymnaea Stagnalis: Motor Control of Shell Turning. Journal of Experimental Biology. 197, 79-99 (1994).
- Jansen, R. F., Pieneman, A. W., Ater Maat, . Pattern Generation in the Buccal System of Freely Behaving Lymnaea stagnalis. Journal of Neurophysiology. 82, 3378-3391 (1999).
- Kupfermann, I. Dissociation of the appetitive and consummatory phases of feeding behavior in Aplysia: a lesion study. Behavioral Biology. 10, 89-97 (1974).
- Scott, M. L., Kirk, M. D. Recovery of consummatory feeding behavior after bilateral lesions of the cerebral-buccal connectives in Aplysia california. Brain Research. 585, 272-274 (1992).
- de Boer, P. A., Jansen, R. F., ter Maat, A., van Straalen, N. M., Koene, J. M. The distinction between retractor and protractor muscles of the freshwater snail’s male organ has no physiological basis. Journal of Experimental Biology. 213, 40-44 (2010).
- Chiel, H. J., Weiss, K. R., Kupfermann, I. An identified histaminergic neuron modulates feeding motor circuitry in Aplysia. Journal of Neuroscience. 6, 2427-2450 (1986).
- Hurwitz, I., Neustadter, D., Morton, D. W., Chiel, H. J., Susswein, A. J. Activity patterns of the B31/B32 pattern initiators innervating the I2 muscle of the buccal mass during normal feeding movements in Aplysia californica. Journal of Neurophysiology. 75, 1309-1326 (1996).
- Kier, W. M. The diversity of hydrostatic skeletons. Journal of Experimental Biology. 215, 1247-1257 (2012).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved