Method Article
Use of Capillary Aerosol Generator in Continuous Production of Controlled Aerosol for Non-Clinical Studies
In This Article
Summary
The protocol describes the settings and use of a capillary aerosol generator for continuous production of controlled aerosol from a multispecies liquid solution, suitable for steady large-volume aerosol delivery (e.g., in vivo inhalation studies).
Abstract
The capillary aerosol generator (CAG) is operated with the principal of thermal liquid evaporation through heating of e-liquid in the initial phase, followed by nucleation and condensation regulated through a mixture of airflow to generate aerosols, such as in an electronic cigarette (EC). The CAG is particularly useful in generating aerosols of large volumes in a continuous manner, for instances such as in vivo inhalation toxicology studies, where usage of ECs is not feasible. The thermal effects of generating aerosol from the CAG are similar in terms of temperature applied in an EC, thus allowing investigators to assess the vapors of e-liquids at scale and reproducibility. As the operation of the CAG allows users to control critical parameters such as the flow rate of e-liquid, heating temperatures and dilution air flows, it allows investigators to test various e-liquid formulations in a well-controlled device. Properties, such as aerosol particle size, are demonstrated to be regulated with the air flow rate with respect to the e-liquid flow and e-liquid composition. The CAG, however, is limited in assessing common EC-related issues, such as overheating of its elements. We seek to demonstrate that the CAG can generate aerosol that is reproducible and continuous, by assessing the chemical and physical aerosol characteristics with a chosen e-liquid formulation. The protocol describes the operating parameters of liquid flow rate, dilution air-flow rates and operating procedures needing to optimize the aerosol concentration and particle size required for an in vivo toxicology study. Presenting the representative results from the protocol and discussing the challenges and applications of working with a CAG, we demonstrate that CAG can be used in a reproducible fashion. The technology and protocol, that has been developed from prior work, serve as a foundation for future innovations for laboratory-controlled aerosol generation investigations.
Introduction
Common e-liquids contain a mixture of propylene glycol, glycerol, water, nicotine, and selected flavors. The composition of an aerosol generated from an EC device depends not only on the liquid formulation, but also on the material, design, and characteristics of the device. Consequently, many EC devices may introduce a large variability in aerosol output1, including device-specific production of elevated levels of unwanted constituents, puff volume variation, change in airflow due to blocked ventilation holes, and "dry puffing" (when the liquid container is nearly empty, causing overheating of the device because part of the delivered energy is not used for liquid evaporation)2. In addition, charging, refilling, and cleaning EC devices during long-term inhalation studies would become a huge additional constraint in terms of logistics3. For these reasons, other aerosol generators should be considered for large-scale production of aerosols and proper evaluation of liquid formulations while avoiding device-related variations in aerosol composition and decreasing the work load4,5. Nevertheless, device-generated aerosols should remain a part of risk-assessment strategies, because the levels of certain constituents in EC devices might be more elevated than those in laboratory-controlled standardized aerosol generators owing to the heating/cooling specificities of the devices6,7,8.
Owing to the limited information on regulatory requirements currently available, evaluation methods for the potential toxicity of aerosols generated by electronic cigarettes (EC) are still evolving9,10,11. However, accurate in vitro and in vivo evaluation requires the generation of well-characterized and reproducible volumes of aerosol over time. Producing aerosol from an EC device with a controlled puffing regimen would certainly be the most representative process from the perspective of user consumption. For regulatory toxicity studies, considering a variety of possible liquid formulations that users may often prepare by themselves and, at the same time, modifying some device characteristics (e.g., delivered energy), the use of EC devices for performing long-term repeated-exposure toxicology studies are not only challenging but also potentially inadequate.
The capillary aerosol generator (CAG)-developed by Philip Morris12,13 and further refined by Virginia Commonwealth University14-works on the principle of creating a jet of hot vapor flow from an electrically heated capillary, that is subsequently cooled down with ambient air, causing particle nucleus formation and subsequent condensation, leading to aerosol formation. Because the same physical processes lead to aerosol formation in ECs (apart from the delivery of the liquid to the capillary by a pump in the CAG, which, in an EC, is usually replaced by capillary forces acting on the wicking material drawing the liquid from the reservoir in the EC), the characteristics of CAG-generated aerosols are very similar to those of EC aerosols14 (Figure 1). The CAG allows production of large volumes of aerosol, with few handling requirements; it is, therefore, particularly suitable for in vivo inhalation studies.
The CAG is a laboratory device consisting of a heated capillary tube simply connected to a temperature controller and to a liquid reservoir via a peristaltic pump (Figure 2A). The capillary (160 mm, 21 G, stainless steel) is heated by four heating elements, all embedded into an aluminum block (Figure 2B). The temperature is typically set at 250-275 °C to mimic the coil-heating conditions of an EC device15. The liquid pumped through the capillary is heated up and turned into hot vapor exiting from the tip of the capillary. The CAG assembly (Figure 2C) requires additional elements for mixing the generated vapor with cold air and forming an aerosol. The abrupt mixing of the hot supersaturated vapor with a cold air stream results in nucleation and subsequent condensation, leading to aerosol formation (Figure 2C). In our CAG design (Figure 3), an additional heated air flow first cools down the external body and afterward circulates along the heating blocks to heat up the air flow, preventing, at the same time, condensation of the liquid backflow at the tip of the capillary and stabilizing the vapor jet burst. Additionally, it creates unwanted shielding of hot vapors, thus impacting the nucleation process. For this reason, the flow rate applied for this air flow should be minimal and fit the purpose of the application. This air flow will be called "heated airflow" throughout this manuscript, although it must be understood that this stream is heated passively by the heating blocks and not on purpose by the user.
The cooling airflow rate has a strong influence on the size of the generated aerosol particles. In aerosol production for in vivo inhalation studies, the dilution air flow will determine the exposure dose and might have to be further diluted before reaching the exposure chamber. Besides the chemical composition of aerosols, it is essential to characterize aerosol particle size distribution (PSD) to ensure that the generated aerosol is similar to that generated by ECs and within the inhalation particle size range recommended by the OECD guidelines (often parameterized by the assumption of log-normality of PSD with mass median aerodynamic diameter [MMAD] and geometric standard deviation [GSD]).
The MMAD of the generated aerosols can vary widely depending on the device design, physicochemical liquid properties of the formulation (e.g., density, viscosity, and surface tension), air flow rate, and temperature dictating thermodynamic conditions14,16,17. For in vivo exposure experiments, the airflow generally consists of conditioned, filtered air at 22 ± 2 °C and 60% ± 5% relative humidity. The generated aerosol can then be diluted further depending on the study requirements, to achieve target concentrations in the test atmosphere. It is then delivered via glass piping to the exposure chamber in order to diminish filtration loss. In the results presented here, the temperature and airflow settings are established to demonstrate that the CAG can be used for continuous production of a controlled aerosol with consistent and inhalable PSD and defined concentrations for in vivo inhalation studies.
In the protocol, we will describe how to: 1) assemble the CAG, 2) determine parameters required to generate aerosol from the CAG, 3) perform aerosol generation, and 4) analyze physical and chemical constituents of interest in the aerosol. For these preliminary runs, we consider a liquid solution based on a mixture of aerosol-forming components: propylene glycol (PG), glycerol (VG), water and nicotine at prescribed mass fractions. Finally, we will share example data for assessment of a complex multispecies mixture generated in our experiments (involving the abovementioned constituents mixed with additional flavor constituents). We will discuss the overall results and challenges along with the applicability of this experimental approach for assessment of such mixtures.
Protocol
1. CAG system assembly
- Assembly of CAG
- Place the capillary in the capillary groove of the aluminum heating blocks, with the output end protruding by about 5 mm.
- Lightly tighten the screws of the two halves of the aluminum heating blocks.
- Assemble the heating elements (a) and thermocouple (b) in the aluminum heating blocks (c), with the wires protruding through the aluminum rear cap (d) (Figure 4A).
- Ensure that the wires of the heating elements are connected to an adaptor and ensure that they are straight.
- Assemble the inner PEEK tube (g) with the outer SS tube (e). Ensure that the 2 x 4 mm push-in fittings (f) are tightly secured onto the outer SS tube (e) (Figure 4B).
- Place O-rings (3 x 30 mm) on the two grooves of the inner PEEK tube (g) and insert the inner PEEK tube (g) into the outer SS tube (e) from the front end.
- Place the assembled aluminum heating elements on the SS rear backing (i), with the aluminum rear cap facing the SS rear backing, and slide the inner PEEK/outer SS tube assembly over the aluminum heating elements through to fit tight with the SS rear backing (i) (Figure 4C).
- Place the aluminum front cap (h) over the aluminum heating element, inside the inner PEEK tube. Ensure that the capillary is slightly protruding from the aluminum front cap. Install the three SS lead screws (j) around the SS rear backing and tighten firmly.
- Place the PEEK adaptor (k) over the inner PEEK tube front. Ensure that the PEEK adaptor fits on the front groove of the inner PEEK tube. Place the 25 mm Scheduler (l) over the PEEK adaptor and through the three SS lead screws. Hand-tighten the nuts over the scheduler such that the PEEK adaptor is tight (Figure 4D).
- Connect the heating elements to the temperature controller and the capillary to the peristaltic pump and the test liquid solution.
- Connect the compressed air for heated air flow to the CAG via the 2 x 4 mm push-in fittings (Figure 4B, [f]).
- Assemble the CAG to the glass piece and connect CAG cooling and first dilution air flows (processed air; Figure 3). Add a second dilution flow entry when necessary as well as aerosol sampling ports and a regulatory T-junction (Figure 5).
- CAG cleaning procedure
- Remove the CAG from the CAG glass assembly setup and clean the glass with dry wipes until the glass is visibly dry.
- Observe the capillary output from the CAG for obstruction. If particle deposition can be observed on the outlet of the capillary, change the capillary. Similarly, upon noticing reduced aerosol delivery, replace the capillary with a new one.
- Disassemble the CAG following steps 1.1.9 to 1.1.1.
- Reassemble the CAG following the steps 1.1.1 to 1.1.9 once the capillary is changed.
2. Calculation of CAG aerosol concentration and dilution
- Theoretical calculation of TDF
- Calculate the TDF based on the concentration of the liquid formulation (called stock solution/concentration here) and the LFR:
TDF: total dilution air flow (L/min)
CStock: stock concentration 2%, w/w)
LFR: liquid Flow Rate (g/min)
CTarget: target concentration (µg/L) - Using a solution with 2% (w/w) nicotine, with a target nicotine aerosol concentration at 15 µg/L and a LFR of 0.35 g/min, assume that 100% yield will be the following:
- Calculate the TDF based on the concentration of the liquid formulation (called stock solution/concentration here) and the LFR:
- Theoretical calculation of LFR
- Calculate the LFR based on the concentration of the liquid stock solution and the TDF:
LFR: liquid flow rate (g/min)
CTarget: target concentration (µg/L)
TDF: total dilution air flow (L/min)
CStock: stock concentration (%, w/w) - Using a solution with 2% (w/w) nicotine, with a target nicotine aerosol concentration at 15 µg/L and a TDF of 300 L/min, assume that a 100% yield will be the following:
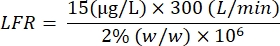
- Calculate the LFR based on the concentration of the liquid stock solution and the TDF:
- Calculation of actual yield (%) based on experimental data
- Based on the above theoretical calculations, perform the initial engineering runs to quantify the actual aerosol constituent concentration (CActual) and obtain the actual yield (AY) of the CAG aerosol. Perform further fine-tuning of aerosol concentration by using the same calculations for adjustment of TDF or LFR.
AY: actual yield (%)
CActual: actual aerosol constituent concentration (µg/L)
TDF: total dilution air flow (L/min)
CStock: stock concentration (%, w/w)
LFR: liquid flow rate (g/min) - Using a solution containing 2% (w/w) nicotine, with a measured nicotine aerosol concentration of 15 µg/L, TDF of 320 L/min, and LFR of 0.35 g/min will result in the following nicotine AY:
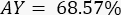
- Based on the above theoretical calculations, perform the initial engineering runs to quantify the actual aerosol constituent concentration (CActual) and obtain the actual yield (AY) of the CAG aerosol. Perform further fine-tuning of aerosol concentration by using the same calculations for adjustment of TDF or LFR.
3. CAG aerosol generation
- Starting aerosol generation
- Weigh and record the value of the test liquid, magnetic stirrer, and the bottle to a nearest 0.01 g. Liquid stock formulations are prepared with components described in Table 1.
- Supply the respective airflow settings (±5%) (Figure 5):
Compressed air for heated flow: 2 L/min
Cooling flow: 10 L/min
First dilution flow: 150 L/min
Second dilution flow: 160 L/min
Waste flow: 172 L/min - Set the temperature control set-point on the digital temperature controller to 250 °C and begin heating of the CAG.
- Place the liquid stock solution with a magnetic stir bar on a magnetic stirrer. Place the inlet tube from the peristaltic pump in the test solution.
- Turn on the peristaltic pump and set the flow to the LFR ±5% (g/min).
- When the CAG temperature reaches 250 ± 1 °C, begin aerosol generation by starting the peristaltic pump to deliver test liquid to the CAG.
- Check whether the aerosol is generated near the capillary tip and record the time as necessary to calculate the mass flow rate. If no aerosol is generated, check all the equipment and settings again. If still no aerosol is generated, it is highly probable that the capillary is blocked and needs to be replaced.
- During aerosol generation
- Drain the liquid that condensates in the glass setup every 60 min, to ensure constant and stable aerosol generation.
- Stopping aerosol generation
- Remove the tubing from the test solution bottle and switch the test liquid to deionized water and record the time for calculating the mass flow rate.
- Wait until water vapor comes out from the capillary, turn off the temperature controller, and keep the peristaltic pump on for at least 10 min to flush and clean the capillary.
- Weigh and record the value of the testing liquid and bottle to the nearest 0.01 g and calculate the mass flow rate using the following equation:
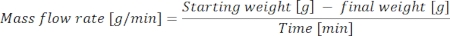
- Turn off the compressed air used as heated flow.
- If necessary, remove the CAG from the assembly setup and clean the glass tubing with dry wipes and reassemble the CAG.
4. Analytical determination of constituents
NOTE: Aerosol sampling is performed at two positions: a) on the undiluted aerosol (both first dilution air and second dilution flow are switched off during undiluted sampling) and b) on the diluted aerosol with all dilutions provided (Figure 5). Up to three sampling ports are available at each of the sampling positions, a and b, allowing simultaneous collection of ACM, and other equipment/probes for analysis of aerosol characteristics. The sampling line is installed perpendicular to the aerosol flow direction and connected to a vacuum pump that allows for drawing a certain volume of aerosol (depending on the pump flow rate and sample duration).
- Determination of Aerosol Collected Mass (ACM)
NOTE: The particulate phase of the aerosol is trapped on a glass-fiber filter pad (diameter: 44 mm, particle size retention: 1.6 µm). ACM weights before and after sampling are measured with filter holders to minimize losses in weighing due to evaporation of volatile components.- Place a filter in the filter holder and place the filter caps.
- Weigh the filter holder to nearest 0.0001 g with the filter before sample collection and document the weight.
- Connect the filter holder containing the filter to the aerosol flow and start sample collection.
- After sample collection, weigh the filter with the filter holder and caps, and document the end weight.
- Calculate the ACM using the following formula:
ACM: concentration of ACM (µg/L)
Wb: weight of the filter and filter holder before sampling (g) to nearest 0.0001 g
Wa: weight of the filter and filter holder after sampling (g) to nearest 0.0001 g
Vaerosol: Volume of aerosol (L) passing through the filter, calculated using:
Sampling time (min) x sampling flow (L/min) - Remove the filter pad from the filter holder and deposit it into a 25 mL glass vial containing 5 mL of ethanol. Extract the ACM by shaking the filter pad on a laboratory shaker for 30 min at 400 rpm.
- Centrifuge the 25 mL glass vial for 5 min at 290 x g and collect the supernatant for quantification of PG/VG and the particulate phase of nicotine.
- Determination of nicotine (or flavor) concentration
NOTE: The aerosol is trapped on a sample column containing specially processed wide-pore diatomaceous earth, a chemically inert matrix for use in a pH range of 1 to 13 (Figure 6).- Prepare the sample column within 15 min before starting the aerosol sample collection.
- For determining nicotine concentrations, add 2 mL of 0.5 M sulfuric acid. For determining flavors, add 2 mL of isopropanol.
- Check the sampling flow.
- Switch on the vacuum pump, and using the calibrated flow equipment that provides accuracy to 1 ccm/min, check the flow rate with a sample column connected to the sampling line.Adjust the flow with the needle valve to the range of 700 ccm/min ± 5%.
- Switch off the vacuum pump.
- Sample collection
- Add the two adaptors to the sample column according to its inlet and outlet side (Figure 6). Connect the tube to the vacuum sampling line via the outlet adaptor.
- Connect the sample column assembly to the sampling port via the inlet adaptor.
- Start sample collection by switching on the vacuum pump.
- Record the sampling start time.
- After a preset sampling time, 10 min at undiluted sampling point A and 30 min at diluted sampling point B, switch off the vacuum pump and record the time.
- Remove the sample column from the sampling port.
- Remove the adaptors from the sample column and seal the sample column with a film membrane to prevent losses due to evaporation or contamination. Label the sample column according to the corresponding sample name.
- Store the sealed sample column in a fridge (2-8 °C) until analysis.
- Determination of carbonyl concentrations
NOTE: Carbonyls are trapped on a glass-filter pad connected in series to a micro-impinger filled with 2,4-dinitrophenylhydrazine (DNPH) dissolved in acetonitrile.
- Prepare the sample column within 15 min before starting the aerosol sample collection.
- Preparation for trapping
- Fill the micro-impinger with 10 mL of 15 mM DNPH in acetonitrile.
- Prepare a filter pad (see paragraph 4.1).
- Check sampling flow
- Switch on the vacuum pump and check the flow rate of the sampling line using a calibrated flow equipment that provides an accuracy of 1 ccm/min. Adjust the flow with the needle valve to the range of 700 ccm/min ± 5%.
- Switch off the vacuum pump.
- Sampling collection
- Connect the filter holder linked to the micro-impinger to the sampling port.
- Connect the vacuum sampling line to the outlet of the micro-impinger.
- Start sample collection by switching on the vacuum pump.
- Record the sampling start time.
- After a preset sampling time, 10 min at undiluted sampling point a and 30 min at diluted sampling point b, switch off the vacuum pump and record the time.
- Disconnect the sampling trap from the sampling port.
- Empty the impinger into a glass vial. Top up the DNPH solution to 10 mL with acetonitrile.
- Determine the weight of the filter pad and extract it in the DNPH-acetonitrile solution by shaking. Discard the filter pad after extraction.
- Take a 1 mL aliquot of the carbonyl-DNPH solution and add 50 µL of pyridine to stabilize the solution.
- Store the aliquots in a freezer at ≤-12 °C until analysis.
- Fill the micro-impinger with 10 mL of 15 mM DNPH in acetonitrile.
Results
Reproducibility of CAG aerosols
To demonstrate the reproducibility of the CAG-generated aerosol, a base liquid solution containing PG, VG, nicotine, water, and ethanol (71.72%, 17.93%, 2%, 5.85%, and 2.5%, respectively) was used over 10 separate aerosol generation runs. The aerosolization and sampling parameters are summarized in Table 2. Chemical characterization of the CAG-generated aerosols confirmed the high degree of reproducibility of the results obtained using the system. Under the same heating, cooling, and dilution air flows as well as the same sampling conditions, the concentrations of ACM, nicotine, VG, and PG were stable over the aerosol generation runs, with the relative standard deviation of 2.48%, 3.28%, 3.43%, and 3.34% of ACM, Nicotine, VG, and PG respectively (Figure 7).
The concentrations of eight carbonyls-namely, acetaldehyde, acetone, acrolein, butyraldehyde, crotonaldehyde, formaldehyde, methyl ethyl ketone, and propionaldehyde-were measured during three consecutive CAG-aerosol generation runs. As expected with aerosols generated at constant controlled conditions, the yields of all carbonyl analytes remained low (Table 3), not reaching the limits of quantification (LOQ) of the analytical method for most compounds. Only acetaldehyde and formaldehyde had yields above the LOQ. Formaldehyde concentrations in the diluted aerosol sample showed high variability (±32%) owing to the volatility of this analyte as well as yields close to the LOQ. The data confirmed the absence of liquid thermal degradation products in CAG-generated aerosols. Addition of a mixture of flavors had an influence on the carbonyl composition of the aerosol. In the present case, acetaldehyde and butyraldehyde yields were drastically increased, from values close to the LOQ to 2.06 and 1.56 µg/L, respectively, in the diluted aerosol meant to enter the exposure chamber. These data highlight the effect of the composition of the flavor mixture on aerosol composition, and stress the need for investigating the potential toxicity of certain flavoring substances in an e-liquid formulation at an early stage, before final assessment in in vivo long-term exposure studies.
PSD of the CAG-generated aerosols
The PSD of the CAG-generated aerosols was measured under different cooling and first dilution flows to evaluate the impact of these conditions on the physical characteristics of the aerosol generated from the base liquid solution containing PG, VG, water, and nicotine only. This procedure is essential for identifying appropriate conditions for producing aerosols with particle sizes in the respirable range.
In the present study, cooling and first dilution flows were modified in steps of 10 L/min to maintain the same total volume of aerosol flow (Table 4). The liquid flow (0.5 mL/min), heated flow (2 L/min), and second dilution flow (150 L/min) were kept constant. Aerosol samples were taken from the diluted sampling point b (Figure 5). PSD was determined by using an aerodynamic particle sizer that measure particle sizes from 0.5 to 20 µm, at a sample flow rate of 5 L/min and diluted appropriately to use with equipment. The MMAD and GSD were reported by the aerodynamic particle sizer for each aerosol generation run.
The increase in cooling flow and simultaneous decrease in first dilution flow had an impact on aerosol particle size (Table 4). The greatest influence on particle size was observed when changing the cooling flow from 10 to 20 L/min and the first dilution flow from 160 to 150 L/min. The MMAD more than doubled under these conditions from 1.47 to 4.03 µm. The average aerosol particle size continued to grow with the increasing cooling flow rates, albeit at lower ratios than those observed between 10 and 20 L/min. The distribution of the aerodynamic diameter of the aerosol particles was clearly shifted toward larger diameters when comparing aerosols generated at 10 L/min cooling flow with those generated at 20-50 L/min (Figure 8).
Trapping efficiency of e-liquid flavors
As discussed earlier, owing to their volatility, various liquid constituents are continuously prone to gas-liquid mass transfer depending on local thermodynamic conditions. In addition, analytical methods have a certain ability to trap such constituents. Actual yield measurements allow us to measure the ability of chemical methods for accurate detection and quantification of selected constituents (for example, because of their condensation potential or reactions, some constituents might not reach their destination, i.e., the exposure chamber in case of inhalation studies). Thus, when assessing various flavored e-liquid formulations, it is essential to be able to determine the most efficient trapping method for chemical assessment of the aerosol. Subsequently, this allows us to measure the transfer rate for each constituent, which is dictated by the often-present losses due to aerosol transport from the place of generation to the exposure chamber. In the present case, an additional study was performed with a liquid containing a mix of flavoring substances. Aerosol was generated with the CAG parameters listed in Table 2 and trapped after dilution (position b, Figure 5), with the sampling flow rate set at 0.7 L/min for 30 min. Trapping was performed on sampling columns preconditioned with 2 mL of isopropanol. The cartridges were eluted with isopropanol shortly after completion of the trapping period, until 20 mL of the solution was recovered. We found that trapping efficiency should generally be investigated and determined for each flavor constituent.
For 70% of the investigated flavor constituents, we had recovery rates >60%, which was well correlated with the boiling points (volatility) of the flavors. This fact implies that inhalation toxicology studies containing complex mixtures should be performed with special attention to the transfer and delivery of aerosol to the exposure site.
Figure 1: Functioning principle of the capillary aerosol generator (CAG). The liquid is pumped into an electrically heated capillary delivering bursts of hot supersaturated vapors, which are cooled down by the air flow, causing sudden nucleation and condensation, leading to aerosol formation. Please click here to view a larger version of this figure.
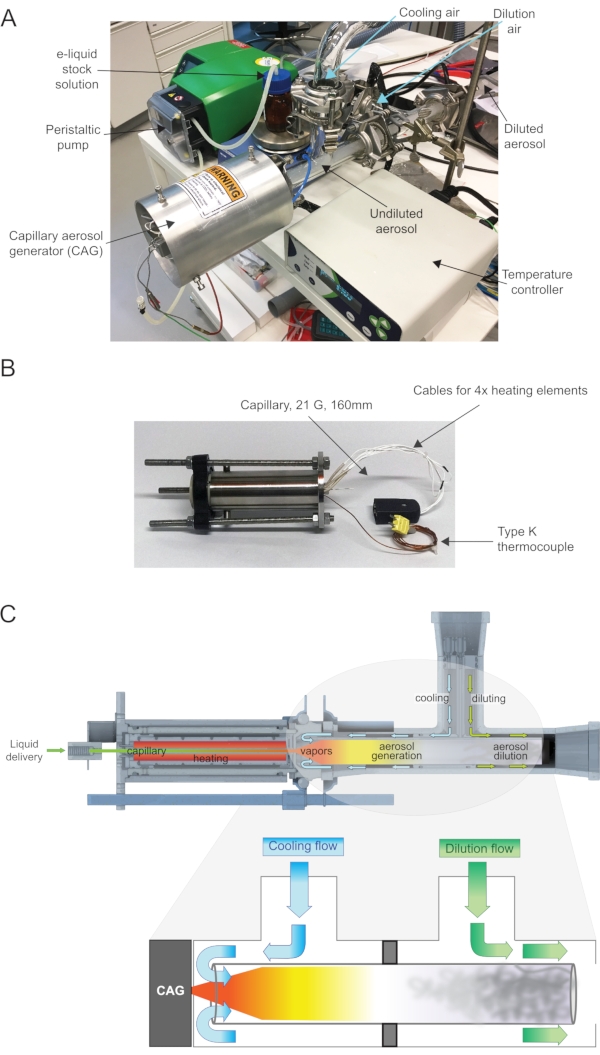
Figure 2: Typical CAG experimental setup and key elements. (A) General view of the CAG assembly, showing the peristaltic pump linking the liquid stock solution to the CAG, dilution air duct, and aerosol formation process. (B) Detailed view of the CAG, with capillary and heating elements. (C) Cross-sectional view of the CAG assembly aerosol generation setup. Details of the cooling and diluting air flows. The glass tubing has two separate compartments. The cooling flow is pushed toward the CAG and enters in contact with the liquid-generated vapor to produce the aerosol. The dilution flow is pushed toward the formed aerosol to dilute the latter. Please click here to view a larger version of this figure.
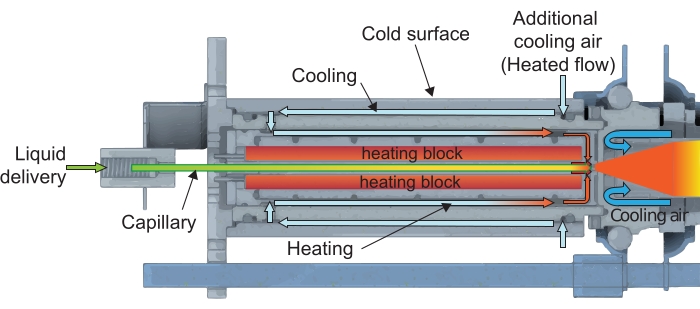
Figure 3: CAG device details: cross-sectional view. The heating flow is introduced around the heating elements for cooling the external CAG body, preventing condensation of the liquid backflow at the tip of the capillary, and for stabilizing the vapor jet burst. Please click here to view a larger version of this figure.
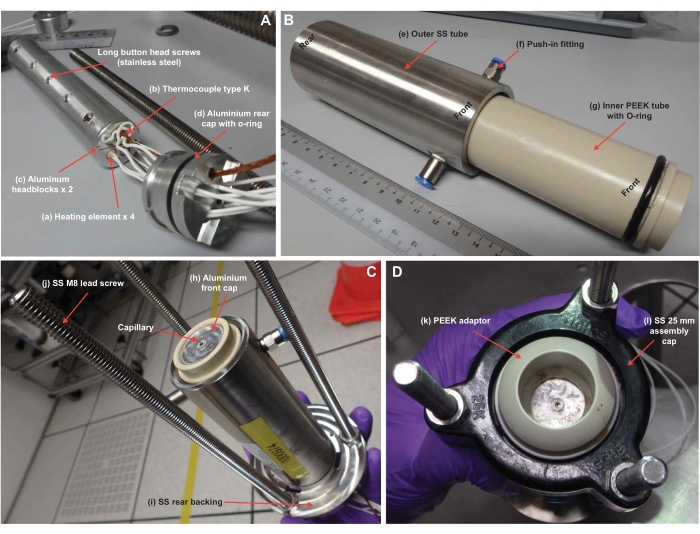
Figure 4: CAG assembly. The capillary and heating element (A) are inserted into an inner PEEK tube, and this assembly is slipped into an outer stainless-steel tube (B). The assembly is capped and tightly fixed on a support using stainless steel lead screws (C,D). The capillary protruding from the rear end is linked via tubing to the peristaltic pump and liquid formulation. Abbreviations: SS, stainless steel. Please click here to view a larger version of this figure.
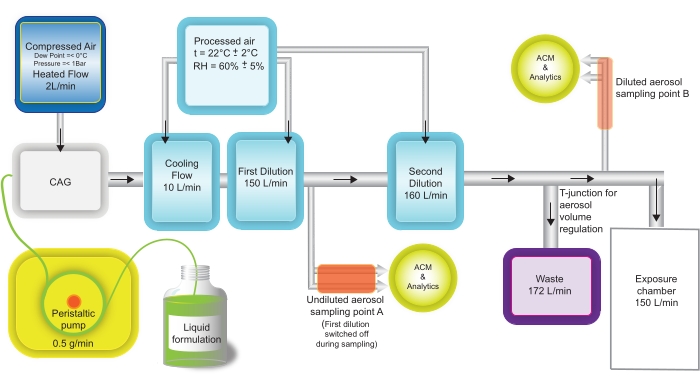
Figure 5: CAG aerosol generation settings for in vivo exposure experiments. Aerosol sampling for analysis takes place at two positions: (a) undiluted aerosol-the first dilution step is switched off during sampling; (b) diluted aerosol, just before entering the exposure chamber. Please click here to view a larger version of this figure.

Figure 6: Sample column with attached adaptors. Before sampling, the sample column is preconditioned with 0.5 M sulfuric acid for nicotine analysis or isopropanol for flavor analysis. The inlet adaptor is connected to the CAG-generated aerosol flow and the outlet adapter to the vacuum pump. Please click here to view a larger version of this figure.
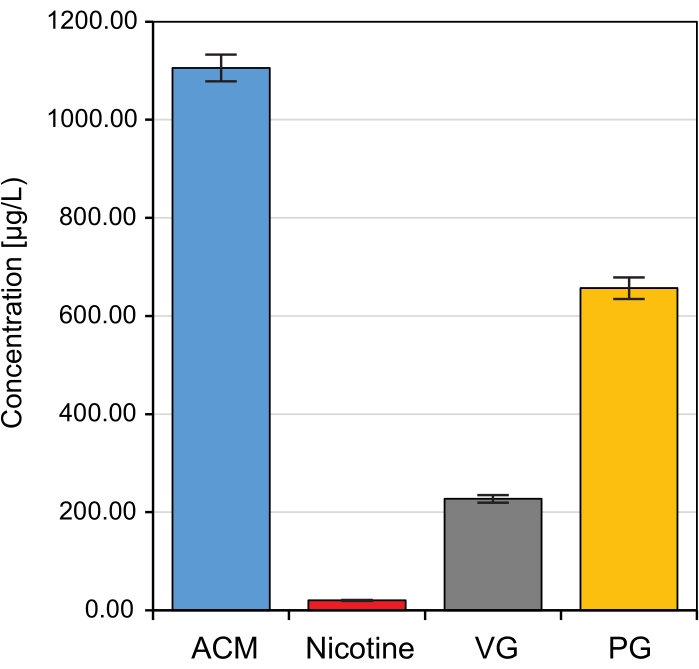
Figure 7: CAG-generated aerosol characterization and reproducibility. Concentration of ACM, nicotine, PG, and VG concentrations over 10 separate experimental aerosol generation runs with the same liquid base solution. ACM, 1105.45 ± 27.4 µg/L; Nicotine, 20.16 ± 0.7 µg/L; VG, 227.15 ± 7.8 µg/L; PG, 656.59 ± 22.0 µg/L. Error bars represent standard deviation. Abbreviations: ACM, aerosol collected mass; PG, propylene glycol; VG, glycerol. Please click here to view a larger version of this figure.
Figure 8: Alterations in particle size distribution of aerosol generated under various cooling flow rates. Please click here to view a larger version of this figure.
| BASE (PG/VG/N) | FLAVOUR (PG/VG/N/F) | |
| Component | PG/VG/N (g/1000g) | PG/VG/N/F (g/1000g) |
| Benzoic Acid | 3.33 | 3.33 |
| PG | 240.00 | 238.91 |
| Water | 150.00 | 150.00 |
| Lactic Acid | 3.33 | 3.33 |
| Acetic Acid | 3.33 | 3.33 |
| Blended Flavor mix | 0.00 | 1.20 |
| Glycerine | 560.01 | 559.90 |
| Nicotine | 40.00 | 40.00 |
| Sum | 1000.00 | 1000.00 |
Table 1: E-liquid stock formulation components18
| Aerosolization protocol | Sampling protocol | ||||
| Parameters | Undiluted | Diluted | Parameters | Undiluted Location A | Diluted Location B |
| CAG temperature (°C) | 250 | ||||
| Pump flow (mL/min) | 0.5 | 0.5 | Sampling time (min) | 10 | 30 |
| Heated air flow (L/min) | 2 | 2 | Sampling flow (ACM) (L/min) | 0.7 | 1.5 |
| Cooling air flow (L/min) | 10 | 10 | Sampling flow Extrelut (L/min) | 0.7 | 0.7 |
| 1st air dilution (L/min) | NA | 150 | Sampling flow Carbonyls (L/min) | 0.7 | 0.7 |
| 2nd air dilution (L/min) | NA | 160 | |||
| Waste (L/min) | NA | 172 | |||
Table 2: Parameters of aerosol generation, dilution, and sampling
| Carbonyls | Base liquid (PG/VG/Nicotine) | Flavor stock solution high concentration with nicotine (PG/VG/Nicotine/Flavors) | ||
| Undiluted aerosol sample µg/L | Diluted aerosol sample µg/L | Undiluted aerosol sample µg/L | Diluted aerosol sample µg/L | |
| Acetaldehyde | 0.834 ± 0.096 | 0.119* | 45.346 ± 1.134 | 2.058 ± 0.202 |
| Acetone | < LOQ | < LOQ | < LOQ | < LOQ |
| Acrolein | < LOQ | < LOQ | < LOQ | < LOQ |
| Butyraldehyde | < LOQ | < LOQ | 36.475 ± 0.996 | 1.557 ± 0.179 |
| Crotonaldehyde | < LOQ | < LOQ | 0.052 ± 0.001 | < LOQ |
| Formaldehyde | 0.731 ± 0.072 | 0.072 ± 0.023 | 0.158 ± 0.007 | 0.026 ± 0.004 |
| Methyl Ethyl Ketone | < LOQ | < LOQ | 0.570 ± 0.015 | < LOQ |
| Propionaldehyde | < LOQ | < LOQ | 0.085 ± 0.001 | < LOQ |
Table 3: Determination of carbonyls in the CAG-generated aerosol. Average values from three aerosol generation runs with the same liquid base solution alone and with a flavor mixture. Only one sample over three runs had values greater than the lower limit of quantification (LOQ) of the method.
| Settings (L/min) | Aerosol droplet diameter | ||
| Cooling flow | 1st dilution flow | MMAD (µm) | GSD |
| 10 | 160 | 1.47 ± 0.04 | 2.07 ± 0.01 |
| 20 | 150 | 4.03 ± 0.18 | 2.13 ± 0.04 |
| 30 | 140 | 4.74 ± 0.04 | 1.89 ± 0.02 |
| 40 | 130 | 5.35 ± 0.04 | 1.80 ± 0.01 |
| 50 | 120 | 5.23 ± 0.03 | 1.76 ± 0.01 |
Table 4: Determination of aerosol particle size (droplet diameter) under different air flow conditions. Abbreviations: MMAD, mass median aerodynamic diameter; GSD, geometric standard deviation.
Discussion
Generating aerosols with CAG helps reduce the variability of EC-device specific aerosolization processes, allowing for objective and controllable assessment of the aerosolized e-liquid formulation itself. CAG-generated aerosols have been shown to be representative of the aerosols generated by ECs7. They can be reproducibly generated with the same composition and characteristics and are, therefore, particularly suitable for in vivo long-term exposure studies requiring large volumes of aerosol over a long period of time8.
The CAG setup is relatively simple to assemble and easy to maintain. However, the operating parameters, such as liquid flow rate and respective air flow rates remain critical for production of controlled aerosol, which requires method optimization according to the purpose of application of the CAG-generated aerosol.
The results presented in the current study show that cooling airflow rate has a clear effect on aerosol particle size distribution. The cooling airflow has a direct impact not only on the nucleation of the generated vapors but also on condensation, because of the cooling of the internal tubing in which the generated aerosol flows. In addition, the dense aerosol is prone to substantial coagulation effects. Combined, these processes are complex and their interaction and influence on aerosol formation are rather difficult to generalize for the specific e-liquids, temperatures, and flows. Supplementary airflow composition (dry or humidified with a fixed percentage of relative humidity)-in particular, water content-will influence heat and mass exchange, leading to not only modulated condensation growth of aerosol particles but also wall condensation. Thus, modifications to this method's parameters are deemed for purpose of use in terms of controlling the PSD17,19.
The presence of chemicals with low solubility or high boiling points could limit the effectiveness of CAG-generated aerosol due to precipitation within the capillary and clogging of the capillary over time. Depending on the chemicals present in the aerosol, the temperature for operating the CAG must be adjusted to generate the vapor. In addition, the stability of the liquid formulation should be regularly assessed. Addition of constituents, including flavors, with different boiling points will have an influence on the final aerosol composition14 and gas-liquid partitioning. It might be necessary to adapt the capillary temperature and heating airflow to prevent backflow and liquid deposition near the hot capillary, which could result in generation of uncontrolled products of thermal degradation (such as carbonyls) because of the long duration of retention of the liquid at a high temperature. In addition, controlling the temperature used to generate the vapor in the capillary has an impact on where the vapor starts to form in the capillary-the higher the temperature, the earlier the vapor is formed. With a higher capillary temperature, the vapor coming out of the capillary will take longer to be cooled down by the cooling air flow and will, therefore, start to nucleate and condense into an aerosol further away from the capillary tip, helping avoid a backflow effect19.
Current e-liquid in vivo toxicology studies are limited in reproducing e-cigarette aerosols due to the logistical complexity to meet the scale of aerosol required, such as in an OECD TG 413 study20. The protocol presented in this study gives an overview on the CAG assembly and settings used at Philip Morris International for aerosol generation in in vivo long-term exposure studies18. These data can serve as a good starting point for further fine-tuning in another laboratory environment (e.g., drug delivery systems21) or for adaptation to specific requirements of a particular study.
Disclosures
The method reported here as well as the specific CAG assembly have been developed for evaluation of aerosols generated from e-liquids to fulfill the requirements of in vivo exposure studies. All the authors are employees of Philip Morris International (PMI) or have worked for PMI under contracted agreements. Philip Morris International is the sole source of funding and sponsor of this study.
Materials
| Name | Company | Catalog Number | Comments |
| Aluminium front cap | Mecanique Buri S.A., La Chaux-de Fonds, Switzerland | Custom Built | Purpose built, 1 x |
| Aluminium heating block, groove diameter 0.4mm | Phil Gunn Machine Co., Inc, VA, USA | B-505432 | 2 x |
| Aluminium rear cap | Mecanique Buri S.A., La Chaux-de Fonds, Switzerland | Custom Built | 1 x |
| Cambridge glass filter pads | GE Healthcare UK Limited | 9703-9654 | 44 mm diameter |
| Capillary 21 G SS, 160 mm | Phil Gunn Machine Co., Inc, VA, USA | 304H21RW | 1 x |
| Dry wipes | Contec Inc. , SC, USA | Prosat Wipes saturated with isopropyl alcohol | cleaning material |
| Flowmeter | TSI, Shoreview, MI, USA | 4100 Series, 0-20 L/min | or equivalent |
| Gilibrator-2 calibrator | Sensidyne, St-Petersburg FL, USA | Gilian Gilibrator-2 | Air flow calibrator |
| Glass Couplings | Labo Service, Kontich, Belgium | QVF | |
| Glass piping | Labo Service, Kontich, Belgium | QVF | Pipe 25 and 40 mm |
| Heating elements | Phil Gunn Machine Co., Inc, VA, USA | LDC01864 | 4 x |
| High heat grease | Lubriplate Lubricant Company, NJ, USA | High temperature multipurpose grease | CAG maintenance |
| Inner PEEK tube | Mecanique Buri S.A., La Chaux-de Fonds, Switzerland | Custom Built | 1 x |
| Magnetic stirrer | IKA-Werke GmbH & Co. KG, Staufen, Germany | C-MAG HS 4 | or equivalent |
| Micro impingers | Labo Service, Kontich, Belgium | Custom Built | |
| Outer SS tube | Mecanique Buri S.A., La Chaux-de Fonds, Switzerland | Custom Built | 1 x |
| PEEK adaptor | Mecanique Buri S.A., La Chaux-de Fonds, Switzerland | Custom Built | Purpose built, 1 x |
| Peristaltic pump | Watson-Marlow Fluid Technology Group, Falmouth, UK | Watson-Marlow 530 U | or equivalent |
| Push-in fitting | Festo Pte Ltd | NPQM-DK-M5-Q4-P10 | 1 x |
| Sample Column Extrelut NT3 cartridge | Merk Sigma-Aldrich | 115095 | |
| SS 25 mm assembly cap | Mecanique Buri S.A., La Chaux-de Fonds, Switzerland | Custom Built | Purpose built, 1 x |
| SS M8 lead screw | Mecanique Buri S.A., La Chaux-de Fonds, Switzerland | Custom Built | 3 x |
| SS M8 nut | Mecanique Buri S.A., La Chaux-de Fonds, Switzerland | Custom Built | 3 x |
| SS rear backing | Mecanique Buri S.A., La Chaux-de Fonds, Switzerland | Custom Built | Purpose built, 1 x |
| Temperature controller | Cole Parmer GmbH, Wertheim, Germany | Digi-Sense TC 9600 | or equivalent |
| Thermocouple type K | RS Components GmbH, Wädenswil, Switzerland | 814-0147 | 1 x |
References
- Williams, M., Talbot, P. Variability among electronic cigarettes in the pressure drop, airflow rate, and aerosol production. Nicotine and Tobacco Research. 13 (12), 1276-1283 (2011).
- Farsalinos, K. E., Voudris, V., Poulas, K. E-cigarettes generate high levels of aldehydes only in 'dry puff' conditions. Addiction. 110 (8), 1352-1356 (2015).
- Werley, M. S., et al. Toxicological assessment of a prototype e-cigaret device and three flavor formulations: a 90-day inhalation study in rats. Inhalation Toxicology. 28 (1), 22-38 (2015).
- Werley, M. S., et al. Non-clinical safety and pharmacokinetic evaluations of propylene glycol aerosol in Sprague-Dawley rats and Beagle dogs. Toxicology. 287 (1-3), 76-90 (2011).
- Werley, M. S., et al. Prototype e-cigarette and the capillary aerosol generator (CAG) comparison and qualification for use in subchronic inhalation exposure testing. Aerosol Science and Technology. 50 (12), 1284-1293 (2016).
- Williams, M., Villarreal, A., Bozhilov, K., Lin, S., Talbot, P. Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS One. 8 (3), 57987 (2013).
- Bekki, K., Uchiyama, S., Ohta, K., Inaba, Y., Kunugita, N. Carbonyl compounds generated from electronic cigarettes. International Journal of Environmental Research and Public Health. 11 (11), 11192-11200 (2014).
- Flora, J. W., et al. Characterization of potential impurities and degradation products in electronic cigarette formulations and aerosols. Regulatory Toxicology and Pharmacology. 74, 1-11 (2016).
- Tobacco Products Directive. Directive 2014/40/EU of the European Parliament and of the Council on 3 April 2014 Available from: https://ec.europa.eu/health/sites/health/files/tobacco/docs/dir_201440_en.pdf (2014)
- Farsalinos, K. E., Le Houezec, J. Regulation in the face of uncertainty: the evidence on electronic nicotine delivery systems (e-cigarettes). Risk Management and Healthcare Policy. 8, 157-167 (2015).
- McNeill, A., Brose, L., Calder, R., Bauld, L., Robson, D. Evidence review of e-cigarettes and heated tobacco products 2018. A report commissioned by Public Health England. Public Health England. , (2018).
- Howell, T. M., Sweeney, W. R. Aerosol and a method and apparatus for generating an aerosol. US Patent. , (1998).
- Dutra, L. M., Grana, R., Glantz, S. A. Philip Morris research on precursors to the modern e-cigarette since 1990. Tobacco Control. 26, 97-105 (2017).
- Gupta, R., Hindle, M., Byron, P. R., Cox, K. A., McRae, D. D. Investigation of a novel Condensation Aerosol Generator: solute and solvent effects. Aerosol Science and Technology. 37 (8), 672-681 (2003).
- Geiss, O., Bianchi, I., Barrero-Moreno, J. Correlation of volatile carbonyl yields emitted by e-cigarettes with the temperature of the heating coil and the perceived sensorial quality of the generated vapours. International Journal of Hygiene and Environmental Health. 219 (3), 268-277 (2016).
- Hong, J. N., Hindle, M., Byron, P. R. Control of particle size by coagulation of novel condensation aerosols in reservoir chambers. Journal of Aerosol Medicine. 15 (4), 359-368 (2002).
- Taylor, G., Warren, S., McRae, D., Venitz, J. Human deposition and exposure studies with propylene glycol aerosols produced using the CAG technology platform. Respiratory Drug Delivery. 1, 183-190 (2006).
- Wong, E. T., et al. A 6-month inhalation toxicology study in Apoe -/- mice demonstrates substantially lower effects of e-vapor aerosol compared with cigarette smoke in the respiratory tract. Archive of Toxicology. 95 (5), 1805-1829 (2021).
- Shen, X., Hindle, M., Byron, P. R. Effect of energy on propylene glycol aerosols using the capillary aerosol generator. International Journal of Pharmaceutics. 275 (1-2), 249-258 (2004).
- Phillips, B., et al. Toxicity of the main electronic cigarette components, propylene glycol, glycerin, and nicotine, in Sprague-Dawley rats in a 90-day OECD inhalation study complemented by molecular endpoints. Food and Chemical Toxicology. 109, 315-332 (2017).
- Hindle, M., Cox, K. A., Gupta, R. Adding pharmaceutical flexibility to the capillary aerosol generator. Proceedings of Respiratory Drug Delivery IX. (Volume III). , 247-253 (2004).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved