Method Article
Manual Construction of a Tissue Microarray using the Tape Method and a Handheld Microarrayer
In This Article
Summary
This protocol outlines the tape method on how to manually construct a tissue microarray using FFPE donor blocks of differing depths.
Abstract
The tissue microarray (TMA) is an important research tool in which many formalin fixed paraffin embedded (FFPE) samples can be represented in a single paraffin block. This is achieved by using tissue cores extracted from the region of interest of different donor FFPE blocks and arranging them into a single TMA paraffin block. Once constructed, sections from the completed TMA can be used to perform immunohistochemistry, chromogenic, fluorescence in situ hybridization (FISH) and RNA ISH studies to assess protein expression as well as genomic and transcriptional alterations in many samples simultaneously, thus minimizing tissue usage and reducing reagent costs. There are several different TMA construction techniques. One of the most common construction methods is the recipient method, which works best with cores of the same length for which a minimum length of 4 mm is recommended. Unfortunately, tissue blocks can be heavily resected during the diagnostic process, frequently resulting in "non-ideal" donor block thicknesses of less than 4 mm. The current article and video focus on the double-sided adhesive tape method; an alternative manual, low cost, easy to use, and rapid method to construct low density (<50 cores) TMAs that is highly compatible with these non-ideal donor blocks. This protocol provides a step-by-step guide on how to construct a TMA using this method, with a focus on the critical importance of pathological review and post construction validation.
Introduction
Formalin fixed paraffin embedded (FFPE) tissues are used extensively in morphological and immunohistochemical protein expression studies1. However, discovery research often requires examination of several markers on a large number of tissues, which can exhaust precious tissues. Introduced in the 1980s, the tissue microarray (TMA) is an important research tool that assembles small exemplary regions of interest from many different FFPE tissue blocks into a single paraffin block, allowing examination of many tissue samples simultaneously2. Thus, TMAs avoid excessive use of highly precious, and often rare, tissue samples while also reducing the costs associated with performing downstream applications on many individual samples3,4.
Several different techniques exist for the construction of TMAs5, including automated and semi-manual approaches6,7. The majority of these latter approaches use the recipient method, wherein tissue cores punched from donor blocks are inserted into a precast mold. However, it is recommended that "ideal" donor blocks that are at least 4 mm thick are used for this method6,7. Unfortunately, donor blocks, particularly those that have been extensively sectioned for clinical diagnostic purposes prior to being made available for research, are frequently less than 4 mm thick, which could exclude them from use in TMA construction using the recipient method, if re-embedding to achieve a depth of 4 mm is not possible or desirable. Moreover, these procedures can often use a benchtop manual tissue microarrayer or expensive automated instruments that are not readily accessible or affordable to the average research laboratory. In contrast, the double-sided adhesive tape method or tape method, is a manual TMA construction method that is compatible with non-ideal donor blocks that uses inexpensive, widely available, reusable or disposable hand-held tissue microarrayers8,9,10. This method inverts the construction process by casting the block around inverted upright cores that upon completion are flush with the top of the TMA, irrespective of core length. As a result, all samples are present in TMA sections when first sectioned, which allows the constructor to get the most out of these non-ideal blocks from the start. Thus, the tape method represents a cost effective and feasible alternative for the non-specialized research laboratories.
TMA construction is not without its challenges, and caution must be taken when selecting the tissue regions to extract the cores from, making pathological review a critical part of the TMA construction process11,12. Thus, this protocol aims to underscore the profound importance of pathological review in TMA construction by highlighting some of the pathological pitfalls associated with TMA construction that individuals constructing and using TMAs should be aware of, and why pathology review should continue through the lifetime of a TMA block.
This protocol outlines the steps taken at the AIDS and Cancer Specimen Resource (ACSR) Technical Core Laboratory to construct TMAs from non-ideal donor blocks using the tape method; where the ACSR is an NIH funded biorepository dedicated to the collection and equitable distribution of biospecimens from HIV cancer tissues in order to promote HIV malignancy research.
Protocol
All donor blocks were deidentified during collection and used for TMA construction in compliance with approved Mayo Clinic IRB protocols (PR16-000507 and PR2207-02).
1. Pathology review
- Retrieve the FFPE tissue donor blocks to be used in the TMA construction.
- Submit a freshly generated hematoxylin and eosin (H&E) stained slide13 for each FFPE tissue donor block selected for histological review by a board-certified pathologist to confirm the diagnosis and annotate the tissue.
NOTE: H&E staining may be performed in house or sent to a pathological core services lab for staining, as was the case in this protocol. One of the most important concepts to remember when constructing TMAs is that the tissues in the FFPE donor blocks are 3-dimensional (3D) structures whose shape, tumor content, and tissue viability can change significantly with increasing block depth. The pathologist must determine, based on the review of the H&E stained tissue section, which is a 2-dimensional representation of the 3D tissue, the best region to extract the tissue core from. - During histological review, ensure that the pathologist identifies and annotates the tissues of interest/not of interest on the H&E stained slides. To annotate the slide, follow the steps below.
- Use a slide marking pen to circle the tissue of interest.
- Using the same marking pen, blot out areas within the circled region that should be avoided.
- Use the marking pen to mark the areas deemed to be ideal for core sampling and extraction.
NOTE: It is important to remember that the tissue shape and composition may change with tissue depth in the block. Thus, the composition of the tissue core may change depending on where the core was extracted, which emphasizes the need for continued pathological guidance. - Submit additional tissue for disease specific immunohistochemical stains alongside the H&Es, if needed, to assist the pathological review. Examples of such stains include ERBB2 staining for HER2 positive breast cancer14, HHV-8 for Kaposi sarcoma15, CD20 for B-cell lymphomas16, U6 as a global marker for RNA quality17, EBER to determine Epstein-Barr virus positivity18, and Vimentin as confirmation of mesenchymal origins and tissue quality control marker19,20.
2. Preparation for TMA construction
- Once the pathology review is complete, compile the final list of donor blocks to be used in the TMA construction and create a TMA map (Figure 1A). The TMA map is a schematic outlining where the cores will be located in the completed TMA and slide mounted tissue samples obtained from the resulting TMA.
- For orientation purposes, ensure that the TMA map avoids placing cores in an even matrix such as a 3 x 3 or 4 x 4 matrix and includes at least 1 orientation marker.
NOTE: Orientation cores can be cores taken from tissue free colored orientation tools21, or tissue blocks containing distinctly different tissues from the theme of the TMA. Unlike the recipient method where upright cores are inserted into a pre-cast wax mold, the tape method creates a TMA by pouring molten wax around inverted, erect cores. This inversion of the construction process requires a second map known as the construction map. - Create the construction map by creating a mirror image of the TMA map (Figure 1B). The construction map shows where each core must be placed during construction in order to appear in the correct location in the completed TMA. Save the construction map.
- Once the maps have been created, prepare the metal TMA base mold. Use a disposable paper checkered grid to guide core placement and regulate core separation.
NOTE: A 6 x 7 (42 spot) template grid for a max of 40 cores (plus for two orientation cores/gaps) for use with commercially available metal base molds with internal dimensions of 26 mm x 20 mm is provided in the supplementary pdf. Print and cut out the paper grid. - Cut the checkered grid to size and affix a piece of double-sided stick tape (DSST) to the back of the grid. Place the grid and DSST tape into the metal tray and add a second piece of DSST on top of the grid, i.e., on top of the paper grid in the metal base mold (Figure 2A).
3. Core placement
- Overlay the pathologically reviewed H&E onto its corresponding tissue block and use the pathologist markings to identify where the tissue block is to be punched (Figure 2B).
- Punch the FFPE donor block using a manual core punch (Figure 2C) at the appropriate place.
NOTE: Core punches are available in a variety of diameters. This method employs a 2 mm diameter handheld core punch.- If using a reusable core punch, ensure that it is cleaned before and after each tissue punch.
- Eject the core from the core punch and use a needle pick to place the ejected core on the crosshairs of the DSST covered grid (Figure 2D). Make sure that when the core is placed on the grid, it is inverted and upright such that the tissue end of the core contacts the DSST (tissue-face-down). Also ensure that the core is placed at the appropriate position on the grid as denoted by the construction map.
- Repeat until all donor blocks are cored and the cores are placed at their appropriate positions.
4. Completing the TMA
- Turn on the bench-top oven, set to 78 °C, and allow sufficient time to get to temperature.
- For each TMA to be constructed, melt 45 g of paraffin pellets in an oven-proof container.
- Label a plastic cassette and place it on top of the metal base mold containing the cores (Figure 2E). The height of the cores should not exceed the depth of the metal tray as tall cores will be tilted or toppled when the cassette is put in place.
- Place the mold with the cassette onto a tray to catch overflow and gently pour the melted paraffin through the cassette into the tray of cores (Figure 2F).
- Allow the molten paraffin to overflow to ensure there are no air bubbles in the body of the TMA. Ensure that the paraffin fills to the top of the cassette so that it is embedded in and firmly bound to the paraffin block once the paraffin has solidified.
- Do not move or disturb the block and allow it to cool at room temperature for 30 min. Then, refrigerate at 4 °C for an additional 30 min to completely solidify.
- Once completely solidified, gently separate the metal base mold from the cassette bound paraffin block of cores.
NOTE: The DSST retains its adhesiveness through the construction process and is frequently attached to the newly formed paraffin block. If applicable, gently remove the DSST and grid from the top of the now completed TMA block.
5. Validating the TMA
- Once the TMA is constructed, use a microtome to section the newly completed TMA. Cut one or more full-faced tissue section.
- Transfer the sections to the prewarmed water bath and slide mount the sections.
NOTE: Newly constructed blocks often require block facing (cutting away of excess paraffin) in order to obtain full face tissue sections that contains all cores. - Once dry, submit a freshly cut slide-mounted TMA section for H&E staining13 and any additional immunohistochemical staining, if needed.
- Submit the stained TMA H&E sections for pathological review.
NOTE: During TMA pathology review, a board-certified pathologist, preferably the same pathologist who reviewed the TMA donor blocks, reviews the TMA H&E stained cores to ensure the desired tissues of interest are indeed present. If any additional tissue or disease-specific immunohistochemical stains were submitted for donor block review in step 1.3.4, these stains should be repeated on TMA sections and submitted alongside the TMA H&E for pathological review. - Record the results of the TMA pathological validation review.
Results
The tape method of TMA construction described here is the method of choice employed at the ACSR Technical Core Laboratory to conserve tissues, which in turn permits frugal and equitable distribution of highly precious tissues to researchers.
A critical component of the construction process is identification of the tissue of interest in a given donor block from where the TMA core should be obtained. This is determined by pathological review wherein a trained pathologist reviews a freshly generated H&E slide (Figure 3). Using a marker, the pathologist circles the area on the H&E slide, which indicates that the core should be obtained from the donor block inside this circle (Figure 3). The pathologist may also mark additional areas such as necrotic areas, which should be avoided, or benign areas from where additional tissue cores may be obtained (Figure 3). Cores are then punched from the indicated areas and included in the construction of the TMA.
There are two principal metrics for successful completion of a TMA by the tape method. The first is the presence of tissue core dots at the expected positions and distances apart from one another, which is assessed by visual inspection. Figure 4A,B depict two successfully completely tape method TMAs and their corresponding H&E slides. Visual inspection of the TMA blocks shows that the cores are present and regularly spaced in each TMA. Some of the principal construction issues that can arise during the tape method construction process include separation of the block and cassette due to premature removal of the metal base prior to wax solidification (Figure 4C). Another potential issue observed with tape method constructed TMAs is core toppling and or placement drift of the embedded cores (Figure 4D); an issue that can arise from excessively turbulent pouring of the molten paraffin, which may be further bolstered by poorly adhesive DSST. Figure 5 shows the H&E images for the cores of TMA1. All but one core (spot 1) is present in the H&E of TMA1. Figure 5 also shows that some cores are present as complete circular tissue dots (i.e., spots 4, 6, 13, 17) while others are not completely present (i.e., spots 3, 8, 9, 12). The tissue loss experienced in these latter cases is not uncommon and may be due to insufficient sectioning needed to reveal all cores in full. Alternatively, the presence of incomplete, or the total absence of tissue (i.e., spot 1), may stem from poor tissue quality of that core, which can result in tissue loss during the staining process.
The second metric of success is assessed in terms of the whether or not the TMA cores captured the tissue of interest. This is achieved via pathological review and validation of the individual TMA H&E cores (Figure 5) compared to the pre-punched donor block H&Es (Figure 3), and where needed/performed any other additional immunohistochemical stains performed. Figure 6 depicts exemplary stains performed on TMA1, including Vimentin, U6, EBER, and CD20. Vimentin positive tissues (brown stain) indicate that all tissues are of mesenchymal origins and are good quality that can be stained. U6 positivity (dark purple/blue stain) indicates that the RNA quality in the tissue is good and compliments RNA in situ hybridization (ISH) stains such as EBER, which target gene transcripts. The U6 results in Figure 5 indicate that RNA quality is high in the lymphoma tissue (spot 9) and the normal tonsil tissue orientation control (spot 22) but not in the normal tissue at spot 19. Following on from this EBER staining was negative in the normal tissue and the orientation control but strongly positive in the lymphoma tissue (spot 9). As expected for tissues of lymphoid origins, both spots 9 and 22 stained positive for the B-cell marker CD20, but spot 19, which stems from normal spinal cord tissue, did not. The staining results for all TMA cores are summarized in Table 1 and confirm the tissue annotation for these TMA tissue cores.
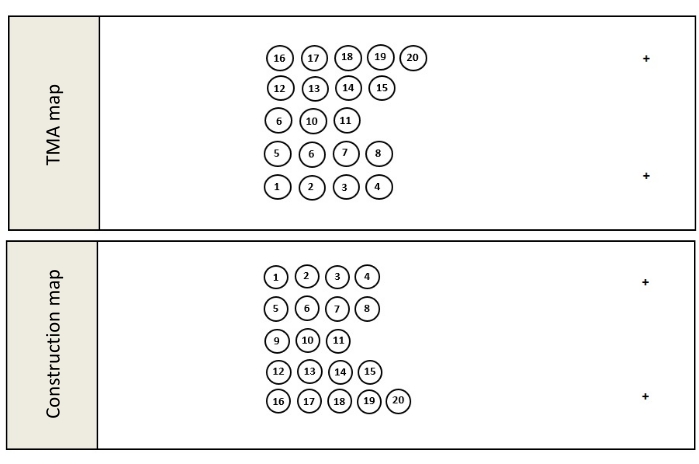
Figure 1: TMA maps. Once all the FFPE blocks and orientation controls have been selected, a detailed map, known as a TMA map (A), outlining where each donor block core will be located in the finished block is created. It is important to note that when constructing a TMA using the tape method, the construction map (B) is a mirror image of the TMA map because this method inverts the construction process, pouring molten paraffin around upright tissue cores rather than inserting the cores into a precast mold. Please click here to view a larger version of this figure.
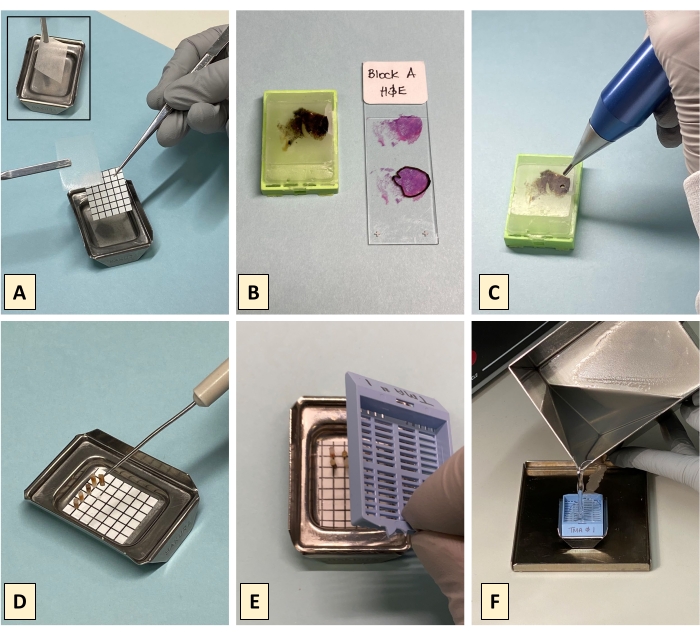
Figure 2: Building the TMA using the tape method. (A) Layer 2 pieces of double-sided stick tape (DSST) and a paper grid in follow order on the bottom of the metal base mold; place the first piece of DSST (2A insert), followed by the disposable paper grid, and then the second piece of DSST at the bottom of the metal base mold. (B) For each donor block, a fresh H&E is generated and reviewed by a pathologist who marks the H&E to denote the tissue of interest, where to punch the block and if applicable where to avoid punching to extract a tissue core. (C) Punch the donor block with a disposal or reusable handheld TMA punch. (D) Using a needle pick, each core is placed standing upright, tumor face down on the DSST covering the grid. (E) Carefully place a pre-labelled plastic cassette on top of the metal tray containing the upright cores. (F) Melted paraffin is gently poured into the tray through the cassette lattice to surround and submerge the upright cores beneath the cassette. Please click here to view a larger version of this figure.
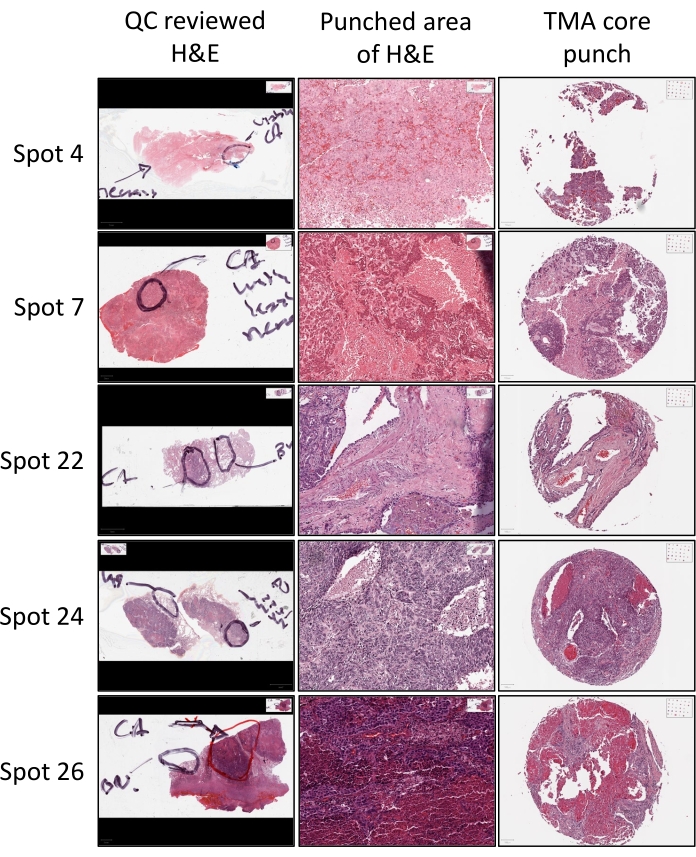
Figure 3: Donor block H&E stain. H&E stained sections from each donor block is subjected to QC review with a training pathologist who annotates the H&E stained slide with a marker to indicate the areas/tissues of interest (i.e., viable cancer (CA) or benign (BN) tissues) for core collection, as well as areas that should be avoided (i.e., necrotic tissue areas). The appropriate areas of the tissue block, as indicated by the annotated H&E are then identified, punched, and incorporated into the TMA being constructed. The resulting TMA core punch H&E can then be referenced back to the unpunched area of the donor block H&E to confirm that the tissue of interest was captured in the core punch. Please click here to view a larger version of this figure.
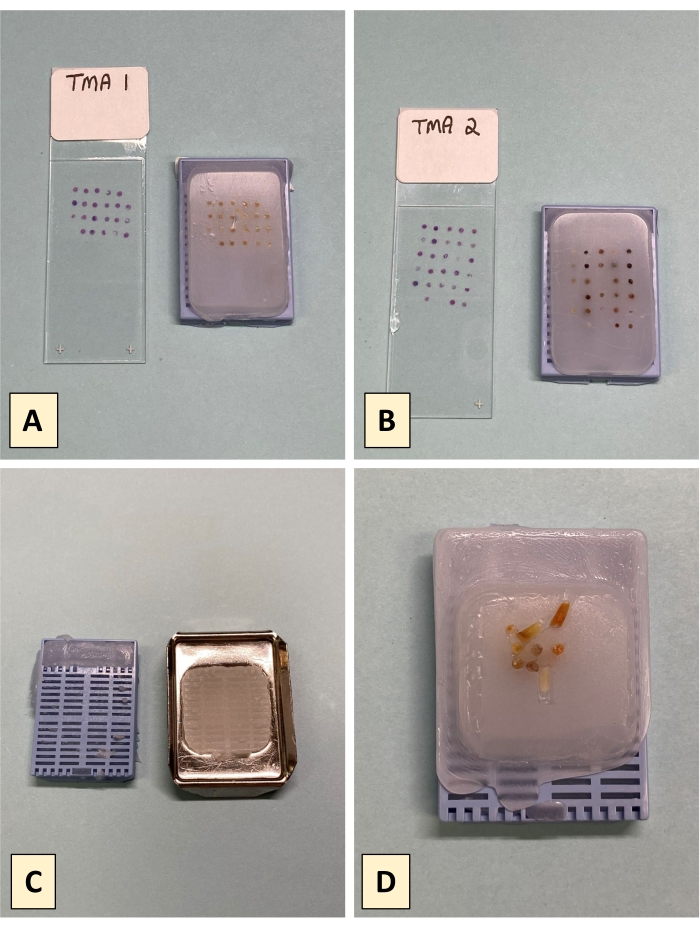
Figure 4: Representative Results. (A,B) Successfully completed tape method TMAs and their accompanying H&Es for pathological review. (C) Separation of the plastic cassette and paraffin block when the cassette is prematurely removed from the metal base mold. (D) Completed tape method TMA showing toppled and migrated cores resulting from turbulent pouring of the molten paraffin. Please click here to view a larger version of this figure.
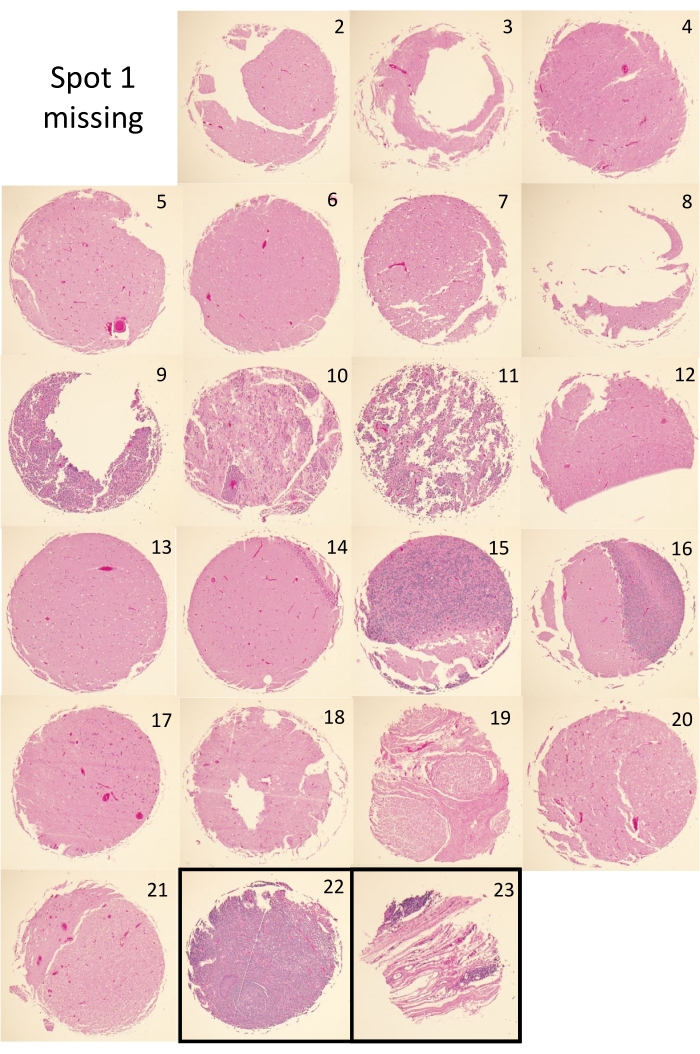
Figure 5: TMA core H&Es. H&E stained section for TMA1, showing the individual H&Es for each tissue core used to construct the TMA (spots 1-21) as well as the orientation cores (spots 22 and 23). Images shown were obtained using a 4x objective, fitted with a digital camera connected to the imaging software. Please click here to view a larger version of this figure.
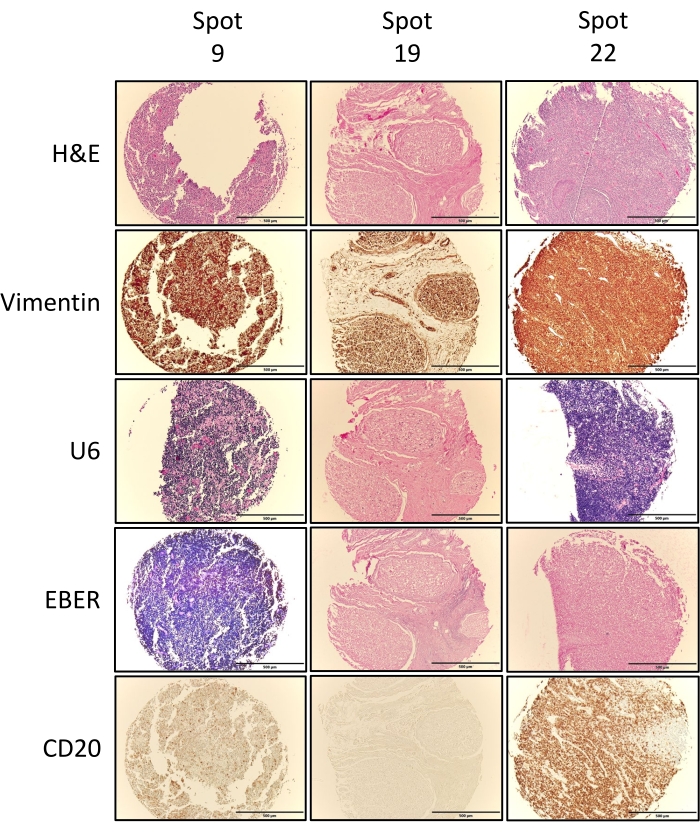
Figure 6: Exemplary immunohistochemical and RNA ISH. Full face sections from TMA1 were assessed by IHC for vimentin and CD20 expression and by RNA ISH to assess RNA quality (U6) and EBV status via EBER ISH. Images show exemplary images for three cores, including one tumor tissue (spot 9), one normal tissue (spot 19), as well as one of the two orientation controls (spot 22). Vimentin positivity is denoted by brown staining, U6 positivity is denoted by blue/purple staining, EBER by blue/purple staining and CD20 by brown staining. Images shown were obtained using a 4x objective, fitted with a digital camera connected to the imaging software. Please click here to view a larger version of this figure.
Table 1: Overview of constructed TMA tissues and stains performed: Table outlines whether or not (a) tissue is present in each of the representative TMA spots (b) tumor is present in each H&E stained tissue spot and (c) the results of any additional immunohistological stains performed on adjacent TMA sections: "-" indicates negative, n/d = not done. Please click here to download this Table.
Supplementary file. Please click here to download this File.
Discussion
One of the most critical components of the TMA construction process is pathology review of FFPE donor blocks from which the TMA cores will be obtained4. During the review, a board-certified pathologist examines a representative H&E stained tissue section from each donor block. It is imperative that the H&E is generated using a freshly cut tissue section so that it is the best representation of its corresponding donor block. The use of older H&Es is not recommended given that FFPE tissues are 3-dimensional structures whose tissue profile can change significantly with block depth and extensive sectioning; this may have occurred since the H&E was generated, potentially making its representation of the FFPE block inaccurate. The review process is essential for the selection of suitable cases and identification of tissue areas from where cores should be obtained, as well as identification of areas that should be avoided when collecting cores. In the absence of pathological review, the probability of including unsuitable tissues significantly increases. Inclusion of such tissues has the potential to render the constructed TMA ineffective and unsuitable for its intended purpose. Importantly, unknowingly using such ineffective TMAs has enormous potential to result in false and misleading data. This combined with the knowledge that the profile of FFPE tissues, and thus their derivative cores, can change significantly with increasing depth highlights the importance of continued pathology review throughout the lifetime of a constructed TMA block. Ideally H&Es should be generated using every 15th or 20th section to ensure any changes in the tissue profiles of the cores are captured and recorded. At a minimum, H&Es should be generated and reviewed at the start and end of a project to monitor these potential changes. In light of these points and the importance of the TMA as a research tool, it is imperative that the pathological review is firmly embedded in the TMA construction process and throughout the life of the TMA block.
FFPE blocks are often extensively sectioned during routine diagnostic processing before being released for research purposes. As a result, donor block depth and thus donor block core lengths are often less than the recipient method ideal of 4 mm. Here we have demonstrated how to construct TMAs using the tape method construction protocol, the principal advantage of which is its compatibility with cores from non-ideal FFPE tissue blocks. Although the tape method is of great research value and offers an inexpensive, convenient, and accessible method for constructing TMA blocks, it is not without its challenges and limitations. Compared to both automated and manual recipient methods, which can accommodate 100-1,000s of cores in single TMA block, a maximum of 40 cores is recommended for TMAs constructed using the tape method9. Another limitation is with respect to the ease of construction. In the recipient method, punched cores are merely inserted into a precast mold, which provides core stability by encasing each core in its own individual well, thereby preventing core migration as well as promoting highly regular core placement and separation22. Moreover, the recipient method offers the optional convenience of being fully manual, semi-manual, and fully automated. In contrast, the manual tape method requires careful, gentle placement of each core by hand using a needle pick. Although the absence of a precast mold in the tape method precludes the highly regular placement and separation experienced with the recipient method, this deficiency is overcome through the inclusion of a checkered grid. It is important that the checkered grid is affixed to the center of the metal tray in order to avoid block-edge placement, which increases the risk of core loss if there is insufficient paraffin holding the core in place. It must also be noted that the small core separations possible with the recipient method cannot be achieved with the tape method due to manual core placement and the need for the needle pick to fit between adjacent cores. Cores are placed in a freestanding erect fashion with the smallest surface area or footprint of the core contacting the DSST covered grid. This setup provides significantly less core stability than the recipient method and confers an enhanced risk for core toppling and or migration when pouring the molten paraffin. Indeed, one of the most critical steps in the protocol is pouring the molten paraffin. It is essential that this is done quickly upon removal from the oven to ensure the paraffin is completely liquid and that the pour is performed gently with minimal turbulence. Interestingly, Chen et al. developed a highly novel auxiliary device, akin to a stencil with 7 x 11 evenly distributed 2 mm diameter holes, that is placed on top of a blank paraffin block to guide needles when creating the recipient block and when inserting the donor block cores23. Although designed to aid recipient block construction, such a device could easily be adapted to the tape method to guide placement, regulate separation, and increase core stability during the construction process.
One of the most significant effectors of core stability is the number of cores included in a tape method TMA. This is because as the number of cores increase, the diameter of the core must reduce in order to accommodate the increasing number of cores, which in turn reduces core footprint adhering to the DSST. A minimum core diameter of 1 mm is recommended for tape method TMA construction, as we have found that cores with smaller diameters are particularly unstable and prone to toppling even with very gentle paraffin pouring. A recent study investigating two different in-house methods that used 16 G needles (core diameter of 1.1 mm) and a 4 mm diameter punch experienced substantial tissue losses with the 1.1 mm (26.5%) but not the 4 mm cores24. This appears to indicate that small cores can be problematic to work with and not just during construction. Moreover, smaller diameters may not represent the original donor block as well as larger cores, making pathological interpretation difficult and increasing the probability of inaccurate donor tissue representation.
The inclusion and placement of orientation blocks is of profound importance in TMA construction. However, this is of particular importance for tape method constructed TMAs. This stems from the fact that the tape method inverts the construction process thereby increasing the risk for spatial disorientation. We advise including up to three orientation cores in every block, and that they are placed away from the sample cores in order to best orient the block. Orientation cores can be cores taken from tissue blocks containing distinctly different tissues from the theme of the constructed TMA or tissue-free-colored orientation tools21, where the latter is particularly helpful for non-pathologists. Combined with non-regular matrix patterned core placement, orientation cores minimize the risk for disorientation.
The pronounced difference in core length between TMAs constructed using the tape and recipient methods stems from inclusion of donor block depth in the decision-making process when selecting the construction method. The protocol outlined here employs a threshold where TMAs are constructed using the tape and recipient methods when the donor blocks have depths of <4 mm and 4 mm, respectively. It is important to note that inclusion of donor block depth in construction method choice is not universal. Although it is possible for TMAs to be constructed using either method irrespective of donor block depth, taller cores can interfere with, and or be toppled or tilted by, the placement of the plastic cassette during TMA construction using the tape method. The choice to include or omit criteria in the decision-making process depends on the amenities available to the laboratory, cost, and the desired final product. Under the parameters of this protocol, the number of slide mounted TMA sections that can be obtained from a tape method constructed TMA is significantly less than that obtained from a recipient method constructed TMA. Although it is possible to re-block FFPE tissues to increase the donor block depth and make them compatible with recipient method, the probability of achieving the same tissue orientation within the re-block is low. In turn, this may require extensive block facing to obtain a full-face section, which would likely include significant tissue loss. After block facing, a tape method constructed TMA yields approximately 50 slide mounted TMA sections with all cores present. However, the exact number will vary from block to block and depends on the length of the cores used to construct the TMA and the thickness of the sections being cut (5 µm versus 4 µm). Moreover, it must also be noted that because of their differing core lengths, the cores will exhaust at different times as the TMA is progressively sectioned; an attribute that reemphasizes the need for continued pathological review.
Although the recipient method offers significant benefits and advantages over the tape method, including a less tedious and more expeditious construction processes, the tape method is not aimed at experienced high throughput laboratories. It is aimed at the average laboratory, particularly those in resource limited settings, with access to donor blocks of variable depths but not to TMA construction services. However, future applications could see automation of this method in order to enhance the pool of eligible samples in high throughput laboratories and eliminate the need for re-blocking of donor blocks. In conclusion, the TMA tape method construction protocol described can be easily established at non-specialized laboratories without the need for expensive equipment. However, it is advised that new users should employ FFPE tissue blocks of no value, tissue free colored orientation tools21 or even colored paraffin blocks with no tissue at first in order to familiarize themselves with the tape method technique before advancing to TMA construction using precious tissues. Although their construction is not without potential pitfalls, which both those constructing and using TMA blocks should be aware of, this seemingly unpolished "homemade" TMA construction method can yield high quality, biologically relevant TMAs for research. Indeed, TMA sections stemming from tape method constructed TMAs are among one of the most requested tissue samples in the ACSR biorepository.
Disclosures
We have nothing to disclose.
Acknowledgements
Funding for this work was provided by the NIH funded AIDS and Cancer Specimen Resource (ACSR) biorepository (www.acsr1.com), UM1CA181255.
Materials
| Name | Company | Catalog Number | Comments |
| BX51 microscope | Olympus | BX51 | |
| cellSens imaging software | Olympus | x | |
| Cotton Balls | FisherBrand | 22-456-880 | |
| Double sided tape (removable) | Scotch | 383534 | |
| DP72 camera | Olympus | DP72 | |
| Economy Lab Oven | FisherBrand | 13246516GAQ | |
| Forceps | Various | x | |
| Formula "R" (paraffin) | Leica | 3801450 | |
| Glass microscope slides | FisherBrand | 12-550-15 | |
| Low Profile Micotome Blades | Accu-Edge | 4689 | |
| Microtome | Leica | RM2265 | |
| Permanent marker | Electron Microscope Sciences | 72109-12 | |
| Quick Ray manual tissue microarrayer set | Unitma | UT06 | |
| Stainless-Steel Base Molds | FisherBrand | 22-038-209 | |
| Tissue Cassette Cooling Tray | Electron Microscope Sciences | 63314 | |
| Tissue Processing/Embedding Cassette | FisherBrand | 15-182-701E | |
| Waterbath | Triangle Biomedical Sciences | TFB-120 | |
| Wooden stick | FisherBrand | 22363158 |
References
- O'Rourke, M. B., Padula, M. P. Analysis of formalin-fixed, paraffin-embedded (FFPE) tissue via proteomic techniques and misconceptions of antigen retrieval. BioTechniques. 60 (5), 229-238 (2016).
- Jawhar, N. M. Tissue microarray: A rapidly evolving diagnostic and research tool. Annals of Saudi Medicine. 29 (2), 123-127 (2009).
- Fowler, C. B., et al. Tissue microarrays: construction and uses. Methods in Molecular Biology. 724, 23-35 (2011).
- Voduc, D., Kenney, C., Nielsen, T. O. Tissue microarrays in clinical oncology. Seminars in Radiation Oncology. 18 (2), 89-97 (2008).
- Vogel, U. Overview on techniques to construct tissue arrays with special emphasis on tissue microarrays. Microarrays (Basel). 3 (2), 103-136 (2014).
- Kampf, C., Olsson, I., Ryberg, U., Sjostedt, E., Ponten, F. Production of tissue microarrays, immunohistochemistry staining and digitalization within the human protein atlas. Journal of Visualized Experiments. (63), e3620 (2012).
- Zlobec, I., Suter, G., Perren, A., Lugli, A. A next-generation tissue microarray (ngTMA) protocol for biomarker studies. Journal of Visualized Experiments. (91), e51893 (2014).
- Pires, A. R., Andreiuolo Fda, M., de Souza, S. R. TMA for all: a new method for the construction of tissue microarrays without recipient paraffin block using custom-built needles. Diagnostic Pathology. 1, 14 (2006).
- Glinsmann-Gibson, B., et al. Recommendations for tissue microarray construction and quality assurance. Applied Immunohistochemistry and Molecular Morphology. 28 (4), 325-330 (2020).
- Wilkens, L. Verfahren und Vorrichtung zur Präparation von Gewebeproben (Method and Apparatus for the Preparation of Tissue Samples). German patent DE. 102, (2003).
- Dancau, A. M., Simon, R., Mirlacher, M., Sauter, G. Tissue microarrays. Methods in Molecular Biology. 1381, 53-65 (2016).
- Bubendorf, L., Nocito, A., Moch, H., Sauter, G. Tissue microarray (TMA) technology: miniaturized pathology archives for high-throughput in situ studies. The Journal of Pathology. 195 (1), 72-79 (2001).
- Feldman, A. T., Wolfe, D. Tissue processing and hematoxylin and eosin staining. Methods in Molecular Biology. 1180, 31-43 (2014).
- Ahn, S., Woo, J. W., Lee, K., Park, S. Y. HER2 status in breast cancer: changes in guidelines and complicating factors for interpretation. Journal of Pathology and Translational Medicine. 54 (1), 34-44 (2020).
- Fukumoto, H., Kanno, T., Hasegawa, H., Katano, H. Pathology of Kaposi's sarcoma-associated herpesvirus infection. Frontiers in Microbiology. 2, 175 (2011).
- Swerdlow, S. H., et al. WHO classification of tumours of haematopoietic and lymphoid tissues. IARC, 4 end. 2, 449-452 (2017).
- Ramsower, C., et al. Assessment of 2-year storage conditions on protein, RNA, and DNA in unstained human tissue sections, including a novel multiplex digital gene expression profiling method with implications for biobanking. Biopreservation and Biobanking. , (2021).
- Weiss, L. M., Chen, Y. Y. EBER in situ hybridization for Epstein-Barr virus. Methods in Molecular Biology. 999, 223-230 (2013).
- Yang, Y., DeYoung, B., Qasem, S. 314 vimentin stain: A useful stain or an ancient change. American Journal of Clinical Pathology. 149, 135 (2018).
- Battifora, H. Assessment of antigen damage in immunohistochemistry. The vimentin internal control. American Journal of Clinical Pathology. 96 (5), 669-671 (1991).
- Coffey, A., Johnson, M. D., Berry, D. L. SpOT the correct tissue every time in multi-tissue blocks. Journal of Visualized Experiments. (99), e52868 (2015).
- Fedor, H. L., De Marzo, A. M. Practical methods for tissue microarray construction. Methods in Molecular Medicine. 103, 89-101 (2005).
- Chen, Y. J., et al. An introduction of an easy-operating and economical technique for tissue microarray preparation. Journal of Clinical Pathology. 73 (7), 403-407 (2020).
- Gomez-de Maria, C., et al. Tissue arrays: Two simple and economical methods for manual construction. Archivos Espanoles de Urologia. 71 (10), 832-839 (2018).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved